Plant dicistronic tRNA-snoRNA genes: a new mode of expression of the small nucleolar RNAs processed by RNase Z
- PMID: 12554662
- PMCID: PMC140725
- DOI: 10.1093/emboj/cdg040
Plant dicistronic tRNA-snoRNA genes: a new mode of expression of the small nucleolar RNAs processed by RNase Z
Abstract
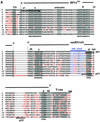
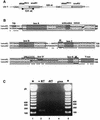
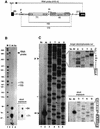
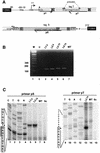
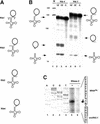
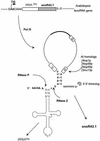
Small nucleolar RNAs (snoRNAs) guiding modifications of ribosomal RNAs and other RNAs display diverse modes of gene organization and expression depending on the eukaryotic system: in animals most are intron encoded, in yeast many are monocistronic genes and in plants most are polycistronic (independent or intronic) genes. Here we report an unprecedented organization: plant dicistronic tRNA-snoRNA genes. In Arabidopsis thaliana we identified a gene family encoding 12 novel box C/D snoRNAs (snoR43) located just downstream from tRNA(Gly) genes. We confirmed that they are transcribed, probably from the tRNA gene promoter, producing dicistronic tRNA(Gly)-snoR43 precursors. Using transgenic lines expressing a tagged tRNA-snoR43.1 gene we show that the dicistronic precursor is accurately processed to both snoR43.1 and tRNA(Gly). In addition, we show that a recombinant RNase Z, the plant tRNA 3' processing enzyme, efficiently cleaves the dicistronic precursor in vitro releasing the snoR43.1 from the tRNA(Gly). Finally, we describe a similar case in rice implicating a tRNA(Met-e) expressed in fusion with a novel C/D snoRNA, showing that this mode of snoRNA expression is found in distant plant species.
Figures

References
-
- Akama K. and Kashihara,M. (1996) Plant nuclear tRNA(Met) genes are ubiquitously interrupted by introns. Plant Mol. Biol., 32, 427–434. - PubMed
-
- Bachellerie J.P. and Cavaillé,J. (1998) Small nucleolar RNAs guide the ribose methylation of eukaryotic rRNAs. In Grosjean,H. and Benne,R. (eds), Modification and Editing of RNA. ASM Press, Washington, DC, pp. 255–272.
-
- Barneche F., Steinmetz,F. and Echeverria,M. (2000) Fibrillarin genes encode both a conserved nucleolar protein and a novel small nucleolar RNA involved in ribosomal RNA methylation in Arabidopsis thaliana. J. Biol. Chem., 275, 27212–27220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

