ATP binding by glutamyl-tRNA synthetase is switched to the productive mode by tRNA binding
- PMID: 12554668
- PMCID: PMC140737
- DOI: 10.1093/emboj/cdg053
ATP binding by glutamyl-tRNA synthetase is switched to the productive mode by tRNA binding
Abstract
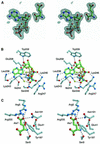
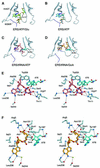
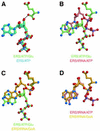
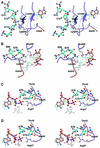
Aminoacyl-tRNA synthetases catalyze the formation of an aminoacyl-AMP from an amino acid and ATP, prior to the aminoacyl transfer to tRNA. A subset of aminoacyl-tRNA synthetases, including glutamyl-tRNA synthetase (GluRS), have a regulation mechanism to avoid aminoacyl-AMP formation in the absence of tRNA. In this study, we determined the crystal structure of the 'non-productive' complex of Thermus thermophilus GluRS, ATP and L-glutamate, together with those of the GluRS.ATP, GluRS.tRNA.ATP and GluRS.tRNA.GoA (a glutamyl-AMP analog) complexes. In the absence of tRNA(Glu), ATP is accommodated in a 'non-productive' subsite within the ATP-binding site, so that the ATP alpha-phosphate and the glutamate alpha-carboxyl groups in GluRS. ATP.Glu are too far from each other (6.2 A) to react. In contrast, the ATP-binding mode in GluRS.tRNA. ATP is dramatically different from those in GluRS.ATP.Glu and GluRS.ATP, but corresponds to the AMP moiety binding mode in GluRS.tRNA.GoA (the 'productive' subsite). Therefore, tRNA binding to GluRS switches the ATP-binding mode. The interactions of the three tRNA(Glu) regions with GluRS cause conformational changes around the ATP-binding site, and allow ATP to bind to the 'productive' subsite.
Figures



References
-
- Brünger A.T. et al. (1998) Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D, 54, 905–921. - PubMed
-
- CCP4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D, 50, 760–763. - PubMed
-
- Cusack S. (1995) Eleven down and nine to go. Nat. Struct. Biol., 2, 824–831. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

