Mouse germ cell-less as an essential component for nuclear integrity
- PMID: 12556490
- PMCID: PMC141152
- DOI: 10.1128/MCB.23.4.1304-1315.2003
Mouse germ cell-less as an essential component for nuclear integrity
Abstract
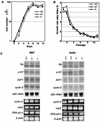
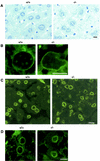
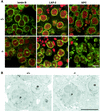
A mouse homologue of the Drosophila melanogaster germ cell-less (mgcl-1) gene is expressed ubiquitously, and its gene product is localized to the nuclear envelope based on its binding to LAP2 beta (lamina-associated polypeptide 2 beta). To elucidate the role of mgcl-1, we analyzed two mutant mouse lines that lacked mgcl-1 gene expression. Abnormal nuclear morphologies that were probably due to impaired nuclear envelope integrity were observed in the liver, exocrine pancreas, and testis. In particular, functional abnormalities were observed in testis in which the highest expression of mgcl-1 was detected. Fertility was significantly impaired in mgcl-1-null male mice, probably as a result of severe morphological abnormalities in the sperm. Electron microscopic observations showed insufficient chromatin condensation and abnormal acrosome structures in mgcl-1-null sperm. In addition, the expression patterns of transition proteins and protamines, both of which are essential for chromatin remodeling during spermatogenesis, were aberrant. Considering that the first abnormality during the process of spermatogenesis was abnormal nuclear envelope structure in spermatocytes, the mgcl-1 gene product appears to be essential for appropriate nuclear-lamina organization, which in turn is essential for normal sperm morphogenesis and chromatin remodeling.
Figures
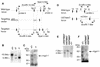



Similar articles
-
Stage-specific expression of mouse germ cell-less-1 (mGCL-1), and multiple deformations during mgcl-1 deficient spermatogenesis leading to reduced fertility.Arch Histol Cytol. 2004 Nov;67(4):335-47. doi: 10.1679/aohc.67.335. Arch Histol Cytol. 2004. PMID: 15700541
-
Synergistic effects of germ cell expressed genes on male fertility in mice.Cytogenet Genome Res. 2003;103(3-4):314-20. doi: 10.1159/000076819. Cytogenet Genome Res. 2003. PMID: 15051954
-
Targeted disruption of the transition protein 2 gene affects sperm chromatin structure and reduces fertility in mice.Mol Cell Biol. 2001 Nov;21(21):7243-55. doi: 10.1128/MCB.21.21.7243-7255.2001. Mol Cell Biol. 2001. PMID: 11585907 Free PMC article.
-
Sperm chromatin protamination: an endocrine perspective.Protein Pept Lett. 2011 Aug;18(8):786-801. doi: 10.2174/092986611795714005. Protein Pept Lett. 2011. PMID: 21443490 Review.
-
The nuclear status of human sperm cells.Micron. 1995;26(4):323-45. doi: 10.1016/0968-4328(95)00007-0. Micron. 1995. PMID: 8574523 Review.
Cited by
-
Transcriptome profiles of Penaeus (Marsupenaeus) japonicus animal and vegetal half-embryos: identification of sex determination, germ line, mesoderm, and other developmental genes.Mar Biotechnol (NY). 2015 Jun;17(3):252-65. doi: 10.1007/s10126-015-9613-4. Epub 2015 Jan 30. Mar Biotechnol (NY). 2015. PMID: 25634056
-
The nuclear form of glutathione peroxidase 4 colocalizes and directly interacts with protamines in the nuclear matrix during mouse sperm chromatin assembly.Spermatogenesis. 2014 Apr 25;4:e28460. doi: 10.4161/spmg.28460. eCollection 2014. Spermatogenesis. 2014. PMID: 25225625 Free PMC article.
-
Regulation of early spermatogenesis in the giant prawn Macrobrachium rosenbergii by a GCL homolog†.Biol Reprod. 2024 May 9;110(5):1000-1011. doi: 10.1093/biolre/ioae028. Biol Reprod. 2024. PMID: 38408206 Free PMC article.
-
Depletion of nucleophosmin leads to distortion of nucleolar and nuclear structures in HeLa cells.Biochem J. 2008 Nov 1;415(3):345-51. doi: 10.1042/BJ20081411. Biochem J. 2008. PMID: 18729828 Free PMC article.
-
The mouse X chromosome is enriched for multicopy testis genes showing postmeiotic expression.Nat Genet. 2008 Jun;40(6):794-9. doi: 10.1038/ng.126. Epub 2008 May 4. Nat Genet. 2008. PMID: 18454149 Free PMC article.
References
-
- Adham, I. M., K. Nayernia, E. Burkhardt-Gottges, O. Topaloglu, C. Dixkens, A. F. Holstein, and W. Engel. 2001. Teratozoospermia in mice lacking the transition protein 2 (Tnp2). Mol. Hum. Reprod. 7:513-520. - PubMed
-
- Alfonso, P. J., and W. S. Kistler. 1993. Immunohistochemical localization of spermatid nuclear transition protein 2 in the testes of rats and mice. Biol. Reprod. 48:522-529. - PubMed
-
- Alsheimer, M., and R. Benavente. 1996. Change of karyoskeleton during mammalian spermatogenesis: expression pattern of nuclear lamin C2 and its regulation. Exp. Cell Res. 228:181-188. - PubMed
-
- Alsheimer, M., E. Fecher, and R. Benavente. 1998. Nuclear envelope remodelling during rat spermiogenesis: distribution and expression pattern of LAP2/thymopopietins. J. Cell Sci. 111:2227-2234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
