Regulation of c-Rel nuclear localization by binding of Ca2+/calmodulin
- PMID: 12556500
- PMCID: PMC141150
- DOI: 10.1128/MCB.23.4.1418-1427.2003
Regulation of c-Rel nuclear localization by binding of Ca2+/calmodulin
Abstract
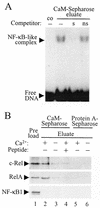
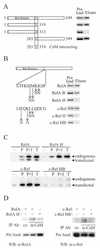
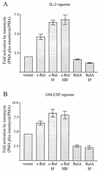
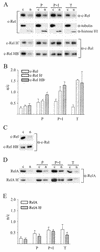
The NF-kappa B/Rel family of transcription factors participates in the control of a wide array of genes, including genes involved in embryonic development and regulation of immune, inflammation, and stress responses. In most cells, inhibitory I kappa B proteins sequester NF-kappa B/Rel in the cytoplasm. Cellular stimulation results in the degradation of I kappa B and modification of NF-kappa B/Rel proteins, allowing NF-kappa B/Rel to translocate to the nucleus and act on its target genes. Calmodulin (CaM) is a highly conserved, ubiquitously expressed Ca(2+) binding protein that serves as a key mediator of intracellular Ca(2+) signals. Here we report that two members of the NF-kappa B/Rel family, c-Rel and RelA, interact directly with Ca(2+)-loaded CaM. The interaction with CaM is greatly enhanced by cell stimulation, and this enhancement is blocked by addition of I kappa B. c-Rel and RelA interact with CaM through a similar sequence near the nuclear localization signal. Compared to the wild-type protein, CaM binding-deficient mutants of c-Rel exhibit increases in both nuclear accumulation and transcriptional activity on the interleukin 2 and granulocyte macrophage colony-stimulating factor promoters in the presence of a Ca(2+) signal. Conversely, for RelA neither nuclear accumulation nor transcriptional activity on these promoters is increased by mutation of the sequence interacting with CaM. Our results suggest that CaM binds c-Rel and RelA after their release from I kappa B and can inhibit nuclear import of c-Rel while letting RelA translocate to the nucleus and act on its target genes. CaM can therefore differentially regulate the activation of NF-kappa B/Rel proteins following stimulation.
Figures



References
-
- Arenzana Seisdedos, F., P. Turpin, M. Rodriguez, D. Thomas, R. T. Hay, J. L. Virelizier, and C. Dargemont. 1997. Nuclear localization of I kappa B alpha promotes active transport of NF-kappa B from the nucleus to the cytoplasm. J. Cell Sci. 110:369-378. - PubMed
-
- Barkett, M., and T. D. Gilmore. 1999. Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene 18:6910-6924. - PubMed
-
- Bird, T. A., K. Schooley, S. K. Dower, H. Hagen, and G. D. Virca. 1997. Activation of nuclear transcription factor NF-kappaB by interleukin-1 is accompanied by casein kinase II-mediated phosphorylation of the p65 subunit. J. Biol. Chem. 272:32606-32612. - PubMed
-
- Capobianco, A. J., D. L. Simmons, and T. D. Gilmore. 1990. Cloning and expression of a chicken c-rel cDNA: unlike p59v-rel, p68c-rel is a cytoplasmic protein in chicken embryo fibroblasts. Oncogene 5:257-265. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
