Disruption of the pelota gene causes early embryonic lethality and defects in cell cycle progression
- PMID: 12556505
- PMCID: PMC141158
- DOI: 10.1128/MCB.23.4.1470-1476.2003
Disruption of the pelota gene causes early embryonic lethality and defects in cell cycle progression
Abstract
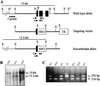
Mutations in either the Drosophila melanogaster pelota or pelo gene or the Saccharomyces cerevisiae homologous gene, DOM34, cause defects of spermatogenesis and oogenesis in Drosophila, and delay of growth and failure of sporulation in yeast. These phenotypes suggest that pelota is required for normal progression of the mitotic and meiotic cell cycle. To determine the role of the pelota in mouse development and progression of cell cycle, we have established a targeted disruption of the mouse PELO: Heterozygous animals are variable and fertile. Genotyping of the progeny of heterozygous intercrosses shows the absence of Pelo(-/-) pups and suggests an embryo-lethal phenotype. Histological analyses reveal that the homozygous Pelo deficient embryos fail to develop past day 7.5 of embryogenesis (E7.5). The failure of mitotic active inner cell mass of the Pelo(-/-) blastocysts to expand in growth after 4 days in culture and the survival of mitotic inactive trophoplast indicate that the lethality of Pelo-null embryos is due to defects in cell proliferation. Analysis of the cellular DNA content reveals the significant increase of aneuploid cells in Pelo(-/-) embryos at E7.5. Therefore, the percent increase of aneuploid cells at E7.5 may be directly responsible for the arrested development and suggests that Pelo is required for the maintenance of genomic stability.
Figures



References
-
- Auer, G. U., T. O. Caspersson, and A. S. Wallgren. 1980. DNA content and survival in mammary carcinoma. Anal. Quant. Cytol. 2:161-165. - PubMed
-
- Basu, J., B. C. Williams, Z. Li, E. V. Williams, and M. L. Goldberg. 1998. Depletion of a Drosophila homolog of yeast Sup35p disrupts spindle assembly, chromosome segregation, and cytokinesis during male meiosis. Cell Motil. Cytoskeleton 39:286-302. - PubMed
-
- Bunz, F., A. Dutriaux, C. Lengauer, T. Waldman, S. Zhou, J. P. Brown, J. M. Sedivy, K. W. Kinzler, and B. Vogelstein. 1998. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science 282:1497-1501. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
