The role of the ubiquitination-proteasome pathway in breast cancer: ubiquitin mediated degradation of growth factor receptors in the pathogenesis and treatment of cancer
- PMID: 12559039
- PMCID: PMC154127
- DOI: 10.1186/bcr541
The role of the ubiquitination-proteasome pathway in breast cancer: ubiquitin mediated degradation of growth factor receptors in the pathogenesis and treatment of cancer
Abstract
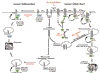
Aberrant activity of growth factor receptors has been implicated in the pathogenesis of a wide variety of malignancies. The negative regulation of signaling by growth factor receptors is mediated in large part by the ubiquitination, internalization, and degradation of the activated receptor. Over the past few years, considerable insight into the mechanisms that control receptor downregulation has been gained. There are also data suggesting that mutations that lead to inhibition of downregulation of growth factor receptors could play a role in the pathogenesis of cancer. Therapies directed at enhancing the degradation of growth factor receptors offer a promising approach to the treatment of malignancies.
Figures
Similar articles
-
The role of the ubiquitination-proteasome pathway in breast cancer: use of mouse models for analyzing ubiquitination processes.Breast Cancer Res. 2003;5(1):16-22. doi: 10.1186/bcr542. Epub 2002 Oct 7. Breast Cancer Res. 2003. PMID: 12559040 Free PMC article. Review.
-
The role of the ubiquitination-proteasome pathway in breast cancer: applying drugs that affect the ubiquitin-proteasome pathway to the therapy of breast cancer.Breast Cancer Res. 2003;5(1):1-7. doi: 10.1186/bcr460. Epub 2002 Aug 14. Breast Cancer Res. 2003. PMID: 12559038 Free PMC article. Review.
-
The ubiquitin-proteasome pathway regulates lysosomal degradation of the growth hormone receptor and its ligand.Biochem Soc Trans. 2001 Aug;29(Pt 4):488-93. doi: 10.1042/bst0290488. Biochem Soc Trans. 2001. PMID: 11498015
-
On and off: proteasome and TGF-beta signaling.Exp Cell Res. 2003 Dec 10;291(2):275-81. doi: 10.1016/j.yexcr.2003.07.007. Exp Cell Res. 2003. PMID: 14644150 Review.
-
Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells.Int J Cancer. 2004 Jan 20;108(3):334-41. doi: 10.1002/ijc.11445. Int J Cancer. 2004. PMID: 14648698
Cited by
-
MicroRNA-mediated regulation of Ubc9 expression in cancer cells.Clin Cancer Res. 2009 Mar 1;15(5):1550-7. doi: 10.1158/1078-0432.CCR-08-0820. Epub 2009 Feb 17. Clin Cancer Res. 2009. PMID: 19223510 Free PMC article.
-
Expression and association of IL-21, FBXL20 and tumour suppressor gene PTEN in laryngeal cancer.Saudi J Biol Sci. 2019 Dec;26(8):2048-2051. doi: 10.1016/j.sjbs.2019.08.013. Epub 2019 Aug 16. Saudi J Biol Sci. 2019. PMID: 31889792 Free PMC article.
-
Identification and analysis of multi-protein complexes in placenta.PLoS One. 2013 Apr 29;8(4):e62988. doi: 10.1371/journal.pone.0062988. Print 2013. PLoS One. 2013. PMID: 23638173 Free PMC article.
-
Hinokitiol inhibits vasculogenic mimicry activity of breast cancer stem/progenitor cells through proteasome-mediated degradation of epidermal growth factor receptor.Oncol Lett. 2016 Apr;11(4):2934-2940. doi: 10.3892/ol.2016.4300. Epub 2016 Mar 2. Oncol Lett. 2016. PMID: 27073579 Free PMC article.
-
The E3 ubiquitin ligase NEDD4 negatively regulates HER3/ErbB3 level and signaling.Oncogene. 2015 Feb 26;34(9):1105-15. doi: 10.1038/onc.2014.56. Epub 2014 Mar 24. Oncogene. 2015. PMID: 24662824
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

