The effects of storage conditions on viability of Clostridium difficile vegetative cells and spores and toxin activity in human faeces
- PMID: 12560391
- PMCID: PMC1769877
- DOI: 10.1136/jcp.56.2.126
The effects of storage conditions on viability of Clostridium difficile vegetative cells and spores and toxin activity in human faeces
Abstract
Aims: Clostridium difficile is a common nosocomial pathogen and as such diagnostic and research methods may necessitate storage of faecal specimens for long periods, followed by subsequent re-examination. This study investigated the effects of storage conditions upon the viability of this organism and its toxin.
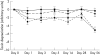
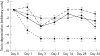
Methods: Three genotypically distinct strains of C difficile (two clinical isolates including the UK epidemic strain, and an environmental isolate) were grown anaerobically at 37 degrees C for 72 hours in a pool of five faecal emulsions. Aliquots of each emulsion were stored at either -20 degrees C (frozen) or 4 degrees C (refrigerated). Emulsions were assayed for viable cells, spores, and cytotoxin titre before storage and at days 1, 3, 5, 7, 14, 28, and 56. An aliquot of each emulsion was also removed, assayed, and replaced in storage at each time point to investigate the effects of multiple freezing/refrigeration/thawing.
Results: Neither storage temperature nor multiple cycles of refrigeration/freezing and thawing adversely affected the viability of C difficile vegetative cells or spores. Single and multiple exposures of samples to 4 degrees C had little effect upon the C difficile toxin titre. Toxin titres of multiply frozen and thawed faeces became significantly lower than for refrigerated faeces (p < 0.01) by day 5 of the experiment in two of the three strains, and in all strains by day 28. Toxin titres of singly frozen faeces became significantly lower than for refrigerated faeces (p < 0.01) by day 56 of the experiment in two of the three strains.
Conclusion: Storage temperature and multiple cycles of freezing (refrigeration)/thawing had minimal effects upon the viability of C difficile or its spores. Storage at 4 degrees C has no discernible effect on C difficile cytotoxin. However, storage at -20 degrees C has a detrimental effect upon C difficile cytotoxin, and multiple cycles of freezing and thawing may further adversely effect toxin titres.
Figures

References
-
- Brazier JS. Role of the laboratory in investigations of Clostridium difficile diarrhoea. Clin Infect Dis 1993;4(suppl):S228–33. - PubMed
-
- Bowman RA, Riley TV. Isolation of Clostridium difficile from stored specimens and comparative susceptibility of various tissue culture cell lines to cytotoxin. FEMS Microbiol Lett 1986;34:31–5.
-
- Borriello SP, Vale T, Brazier JS, et al. Evaluation of a commercial enzyme immunoassay for the detection of Clostridium difficile toxin A. Eur J Clin Microbiol Infect Dis 1988;7:476–84. - PubMed
-
- Weese JS, Staempfli HR, Prescott JF. Survival of Clostridium difficile and its toxins in equine feces: implications for diagnostic test selection and interpretation. J Vet Diagn Invest 2000;12:332–6. - PubMed
-
- Wilcox MH, Fawley WN, Parnell P. Value of lysozyme agar incorporation and alkaline thioglycollate exposure for the environmental recovery of Clostridium difficile. J Hosp Infect 2000;44:65–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
