Roles for CD40, B7 and major histocompatibility complex in induction of enhanced immunity by cryptococcal polysaccharide-pulsed antigen-presenting cells
- PMID: 12562324
- PMCID: PMC1782881
- DOI: 10.1046/j.1365-2567.2003.01574.x
Roles for CD40, B7 and major histocompatibility complex in induction of enhanced immunity by cryptococcal polysaccharide-pulsed antigen-presenting cells
Abstract
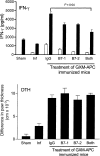
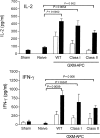
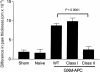
Immunization of mice with activated antigen-presenting cells (APC) pulsed ex vivo with cryptococcal capsular polysaccharide, a glucuronoxylomannan (GXM-APC) results in prolongation of survival and delayed-type hypersensitivity (DTH) responsiveness following infection with Cryptococcus neoformans (NU-2). GXM-APC has both non-specific and GXM-specific effects that influence the immune responses that develop in mice after infection with NU-2. Type 1 cytokine responses are augmented after immunization with APC alone, while GXM must be present for the vaccine to influence survival and DTH reactions. This investigation evaluated the role that major histocompatibility complex (MHC) and co-stimulatory molecules play in the non-specific and GXM-specific responses induced by GXM-APC. APC from CD40 knockout mice were as effective as wild-type APC for the induction of non-specific and GXM-specific responses. Blocking activity of B7-1 and B7-2 by treatment of immunized mice with monoclonal antibodies specific for these molecules just before and for 6 days following GXM-APC immunization decreased the splenic interferon-gamma response of mice subsequently infected with NU-2, but only in mice that were treated with both antibodies. These antibody treatments had no effect on DTH reactivity in similarly treated animals. MHC class I molecules were not involved in the antigen non-specific or GXM-specific activities of the vaccine. MHC class II molecules were not required for augmentation of type 1 cytokine responses but were needed for induction of the GXM-specific response that regulates the expression of DTH reactivity. This investigation has shown that an MHC class II-restricted, GXM-specific response is responsible for altering DTH responsiveness which is the correlate of immunity in this model.
Figures

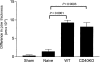
Similar articles
-
Regulation of cytokine expression in mice immunized with cryptococcal polysaccharide, a glucuronoxylomannan (GXM), associated with peritoneal antigen-presenting cells (APC): requirements for GXM, APC activation, and interleukin-12.Infect Immun. 2000 Sep;68(9):5146-53. doi: 10.1128/IAI.68.9.5146-5153.2000. Infect Immun. 2000. PMID: 10948138 Free PMC article.
-
Presentation of cryptococcal capsular polysaccharide (GXM) on activated antigen-presenting cells inhibits the T-suppressor response and enhances delayed-type hypersensitivity and survival.Immunology. 1997 Nov;92(3):334-9. doi: 10.1046/j.1365-2567.1997.00357.x. Immunology. 1997. PMID: 9486105 Free PMC article.
-
Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses.J Immunol. 2000 Jul 1;165(1):158-67. doi: 10.4049/jimmunol.165.1.158. J Immunol. 2000. PMID: 10861048
-
Polysaccharides, mimotopes and vaccines for fungal and encapsulated pathogens.Trends Microbiol. 2001 Sep;9(9):445-51. doi: 10.1016/s0966-842x(01)02134-5. Trends Microbiol. 2001. PMID: 11553457 Review.
-
Mechanism of action of antibody to capsular polysaccharide in Cryptococcus neoformans infection.Front Biosci. 1998 Feb 1;3:d136-51. doi: 10.2741/a270. Front Biosci. 1998. PMID: 9445465 Review.
Cited by
-
Paradoxical Immune Responses in Non-HIV Cryptococcal Meningitis.PLoS Pathog. 2015 May 28;11(5):e1004884. doi: 10.1371/journal.ppat.1004884. eCollection 2015 May. PLoS Pathog. 2015. PMID: 26020932 Free PMC article.
-
The capsule of the fungal pathogen Cryptococcus neoformans.Adv Appl Microbiol. 2009;68:133-216. doi: 10.1016/S0065-2164(09)01204-0. Adv Appl Microbiol. 2009. PMID: 19426855 Free PMC article. Review.
References
-
- Blackstock R, Hernandez NC. Inhibition of phagocytosis in cryptococcosis: Phenotypic analysis of the suppressor cell. Cell Immunol. 1988;114:174–87. - PubMed
-
- Blackstock R, Zembala M, Asherson GL. Functional equivalence of cryptococcal and haptene-specific T-suppressor factor (TsF) 2. Monoclonal anti-cryptococcal TsF inhibits both phagocytosis by a subset of macrophages and transfer of contact sensitivity. Cell Immunol. 1991;136:448–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

