Recognition of bovine respiratory syncytial virus proteins by bovine CD8+ T lymphocytes
- PMID: 12562331
- PMCID: PMC1782889
- DOI: 10.1046/j.1365-2567.2003.01566.x
Recognition of bovine respiratory syncytial virus proteins by bovine CD8+ T lymphocytes
Abstract
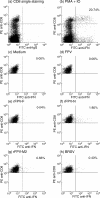
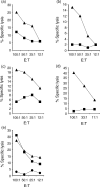
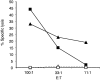
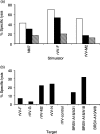
CD8+ T lymphocytes play a major role in the clearance of bovine respiratory syncytial virus (BRSV), an important respiratory pathogen of young calves that shares many of the epidemiological and pathological features of human respiratory syncytial virus (HRSV) in infants. Recombinant vaccinia virus (rVV) and recombinant fowlpox virus (rFPV), expressing individual BRSV proteins, were used to demonstrate that the F, N and M2 proteins were the major antigens recognized by bovine CD8+ T cells in major histocompatibility complex (MHC)-defined cattle. BRSV protein recognition by CD8+ T cells was analysed using cytotoxic T lymphocyte (CTL) assays or by the production of interferon-gamma (IFN-gamma) following restimulation with BRSV proteins. Strong recognition of the G protein by CD8+ T cells was observed in cattle that had been vaccinated with rVV expressing this protein and subsequently challenged with BRSV. Although there is variation in the number of expressed MHC genes in cattle with different class I haplotypes, this did not appear to influence BRSV protein recognition by CD8+ T cells. Knowledge of the antigenic specificity of BRSV-specific CD8+ T cells will facilitate the qualitative and quantitative analysis of BRSV-specific CD8+ T-cell memory in cattle and help to ensure that potential vaccines induce a qualitatively appropriate CD8+ T-cell response.
Figures
References
-
- Stott EJ, Taylor G. Respiratory syncytial virus. Brief review. Arch Virol. 1985;84:1–52. - PubMed
-
- Elvander M. Severe respiratory disease in dairy cows caused by infection with bovine respiratory syncytial virus. Vet Rec. 1996;138:101–5. - PubMed
-
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34. - PubMed
-
- Schreiber P, Matheise JP, Dessy F, Heimann M, Letesson JJ, Coppe P, Collard A. High mortality rate associated with bovine respiratory syncytial virus (BRSV) infection in Belgian white blue calves previously vaccinated with an inactivated vaccine. J Vet Med B, Infectious Dis Vet Public Health. 2000;47:535–50. - PubMed
-
- Anderson LJ, Heilman CA. Protective and disease-enhancing immune responses to respiratory syncytial virus. J Infect Dis. 1995;171:1–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

