Dendritic cells infected with adenovirus expressing the thyrotrophin receptor induce Graves' hyperthyroidism in BALB/c mice
- PMID: 12562382
- PMCID: PMC1808615
- DOI: 10.1046/j.1365-2249.2003.02080.x
Dendritic cells infected with adenovirus expressing the thyrotrophin receptor induce Graves' hyperthyroidism in BALB/c mice
Abstract
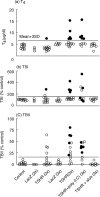
Dendritic cells (DCs) are the most potent antigen-presenting cells and a prerequisite for the initiation of primary immune response. This study was performed to investigate the contribution of DCs to the initiation of Graves' hyperthyroidism, an organ-specific autoimmune disease in which the thyrotrophin receptor (TSHR) is the major autoantigen. DCs were prepared from bone marrow precursor cells of BALB/c mice by culturing with granulocyte macrophage-colony stimulating factor and interleukin-4. Subcutaneous injections of DCs infected with recombinant adenovirus expressing the TSHR (but not beta-galactosidase) in syngeneic female mice induced Graves'-like hyperthyroidism (8 and 35% of mice after two and three injections, respectively) characterized by stimulating TSHR antibodies, elevated serum thyroxine levels and diffuse hyperplasitc goiter. TSHR antibodies determined by ELISA were of both IgG1 (Th2-type) and IgG2a (Th1-type) subclasses, and splenocytes from immunized mice secreted interferon-gamma (a Th1 cytokine), not interleukin-4 (a Th2 cytokine), in response to TSHR antigen. Surprisingly, IFN-gamma secretion, and induction of antibodies and disease were almost completely suppressed by co-administration of alum/pertussis toxin, a Th2-dominant adjuvant, whereas polyriboinosinic polyribocytidylic acid, a Th1-inducer, enhanced splenocyte secretion of IFN-gamma without changing disease incidence. These observations demonstrate that DCs efficiently present the TSHR to naive T cells to induce TSHR antibodies and Graves'-like hyperthyroidism in mice. In addition, our results challenge the previous concept of Th2 dominance in Graves' hyperthyroidism and provide support for the role of Th1 immune response in disease pathogenesis.
Figures




Similar articles
-
TSH receptor-adenovirus-induced Graves' hyperthyroidism is attenuated in both interferon-gamma and interleukin-4 knockout mice; implications for the Th1/Th2 paradigm.Clin Exp Immunol. 2004 Dec;138(3):417-22. doi: 10.1111/j.1365-2249.2004.02641.x. Clin Exp Immunol. 2004. PMID: 15544617 Free PMC article.
-
Schistosoma mansoni and alpha-galactosylceramide: prophylactic effect of Th1 Immune suppression in a mouse model of Graves' hyperthyroidism.J Immunol. 2004 Aug 1;173(3):2167-73. doi: 10.4049/jimmunol.173.3.2167. J Immunol. 2004. PMID: 15265954
-
Prevention of autoantibody-mediated Graves'-like hyperthyroidism in mice with IL-4, a Th2 cytokine.J Immunol. 2003 Apr 1;170(7):3522-7. doi: 10.4049/jimmunol.170.7.3522. J Immunol. 2003. PMID: 12646613
-
Insight into Graves' hyperthyroidism from animal models.Endocr Rev. 2005 Oct;26(6):800-32. doi: 10.1210/er.2004-0023. Epub 2005 Apr 12. Endocr Rev. 2005. PMID: 15827111 Review.
-
Animal models of Graves' hyperthyroidism.Autoimmunity. 2003 Sep-Nov;36(6-7):381-7. doi: 10.1080/08916930310001602966. Autoimmunity. 2003. PMID: 14669945 Review.
Cited by
-
Cancer immunotherapy using dendritic/tumour-fusion vaccine induces elevation of serum anti-nuclear antibody with better clinical responses.Clin Exp Immunol. 2006 Apr;144(1):41-7. doi: 10.1111/j.1365-2249.2006.03029.x. Clin Exp Immunol. 2006. PMID: 16542363 Free PMC article.
-
Attenuation of induced hyperthyroidism in mice by pretreatment with thyrotropin receptor protein: deviation of thyroid-stimulating to nonfunctional antibodies.Endocrinology. 2009 Aug;150(8):3944-52. doi: 10.1210/en.2009-0181. Epub 2009 Apr 23. Endocrinology. 2009. PMID: 19389831 Free PMC article.
-
Evidence that factors other than particular thyrotropin receptor T cell epitopes contribute to the development of hyperthyroidism in murine Graves' disease.Clin Exp Immunol. 2004 Mar;135(3):391-7. doi: 10.1111/j.1365-2249.2004.02399.x. Clin Exp Immunol. 2004. PMID: 15008970 Free PMC article.
-
Breaking tolerance to thyroid antigens: changing concepts in thyroid autoimmunity.Endocr Rev. 2014 Feb;35(1):59-105. doi: 10.1210/er.2013-1055. Epub 2013 Dec 4. Endocr Rev. 2014. PMID: 24091783 Free PMC article. Review.
-
Evidence that TSH Receptor A-Subunit Multimers, Not Monomers, Drive Antibody Affinity Maturation in Graves' Disease.J Clin Endocrinol Metab. 2015 Jun;100(6):E871-5. doi: 10.1210/jc.2015-1528. Epub 2015 Apr 9. J Clin Endocrinol Metab. 2015. PMID: 25856215 Free PMC article.
References
-
- Rees Smith B, McLachlan SM, Furmaniak J. Autoantibodies to the thyrotropin receptor. Endocr Rev. 1988;9:106–21. - PubMed
-
- Rapoport B, Chazenbalk GD, Jaume JC, et al. The thyrotropin (TSH) receptor: interaction with TSH and autoantibodies. Endocr Rev. 1998;19:673–716. - PubMed
-
- Bottazzo GF, Pujol-Borrel R, Hanafusa T, et al. Role of aberrant HLA-DR expression and antigen presentation in induction of endocrine autoimmunity. Lancet. 1983;ii:1115–8. - PubMed
-
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

