High circulating levels of biologically inactive IL-6/SIL-6 receptor complexes in systemic juvenile idiopathic arthritis: evidence for serum factors interfering with the binding to gp130
- PMID: 12562400
- PMCID: PMC1808632
- DOI: 10.1046/j.1365-2249.2003.02052.x
High circulating levels of biologically inactive IL-6/SIL-6 receptor complexes in systemic juvenile idiopathic arthritis: evidence for serum factors interfering with the binding to gp130
Abstract
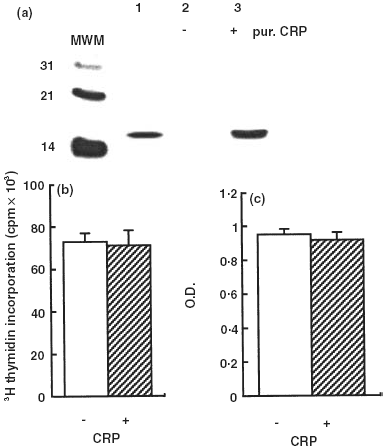
We previously demonstrated that high levels of IL-6/sIL-6R complexes are present in sera of patients with systemic juvenile idiopathic arthritis (s-JIA) and that the amount of IL-6 estimated in the IL-6/sIL-6R complexes is markedly higher than that measured by the B9 assay. Here, we show that two additional bioassays, employing human myeloma XG-1 cells and human hepatoma Hep3B cells, detected serum IL-6 levels similar to those measured by the B9 assay and approximately 10-fold lower than the IL-6 levels estimated to be present in the IL-6/sIL-6R complex. Using an assay for the measurement of the amount of circulating IL-6 complexed with the sIL-6R and available for binding to gp130 (gp130 binding activity), we show that the IL-6/gp130 binding activity is similar to that detected by the bioassays and again significantly lower than that estimated to be present in the IL-6/sIL-6R complex. Addition of recombinant human IL-6 (rhIL-6) to sera of patients or controls results in a markedly lower increase in the gp130 binding activity in patients than in controls. Moreover, sera from s-JIA patients inhibited in a dose dependent manner the gp130 binding activity assay. These results show that sera from patients with s-JIA contain a factor, or factors, that inhibit(s) the binding of the IL-6/sIL-6R complex to gp130. This inhibitory activity does not appear to be due to soluble gp130, C-reactive protein or autoantibodies to IL-6.
Figures
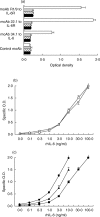
 ) or in the presence of rhsIL-6R alone (100 ng/ml,
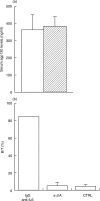
) or in the presence of rhsIL-6R alone (100 ng/ml,  ;), or of rhsIL-6R (100 ng/ml) and rhIL-6 (3 ng/m)(h) and then added to wells coated with the indicated mAb. j None. The IL-6/sIL-6R complex bound to gp130FLAG was revealed with a mAb to the FLAG epitope. Results are shown as total O.D., as means + SE, represented by the horizontal bars, of triplicate determinations. (b) Dose–response curves of the assay for the IL-6/gp130 binding activity obtained by adding increasing concentrations of rhIL-6 to rhsIL-6R (100 ng/ml) (
;), or of rhsIL-6R (100 ng/ml) and rhIL-6 (3 ng/m)(h) and then added to wells coated with the indicated mAb. j None. The IL-6/sIL-6R complex bound to gp130FLAG was revealed with a mAb to the FLAG epitope. Results are shown as total O.D., as means + SE, represented by the horizontal bars, of triplicate determinations. (b) Dose–response curves of the assay for the IL-6/gp130 binding activity obtained by adding increasing concentrations of rhIL-6 to rhsIL-6R (100 ng/ml) ( ) or to a normal human serum (s) in the presence of gp130FLAG (250 ng/ml). Reaction mixtures were incubated at 37°C for 1 h and then tested, as described in the methods section, in the assay for IL-6/gp130 activity using the mAb 22·1 to the sIL-6R for capture. Results are shown as specific O.D., as means + SE, represented by the vertical bars, of triplicate determinations. (c) Dose–response curves of the assay for the IL-6/gp130 binding activity obtained by reading the A405 after a 30 minute (j), a 2-h (▴) and a 6-h (d) incubation with the substrate p-NPP. Results are shown as specific O.D., as means and SE, represented by the vertical bars, of triplicate determinations.
) or to a normal human serum (s) in the presence of gp130FLAG (250 ng/ml). Reaction mixtures were incubated at 37°C for 1 h and then tested, as described in the methods section, in the assay for IL-6/gp130 activity using the mAb 22·1 to the sIL-6R for capture. Results are shown as specific O.D., as means + SE, represented by the vertical bars, of triplicate determinations. (c) Dose–response curves of the assay for the IL-6/gp130 binding activity obtained by reading the A405 after a 30 minute (j), a 2-h (▴) and a 6-h (d) incubation with the substrate p-NPP. Results are shown as specific O.D., as means and SE, represented by the vertical bars, of triplicate determinations.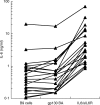
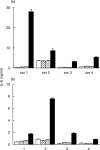
 ), by the IL-6/gp130 binding activity assay (
), by the IL-6/gp130 binding activity assay ( ) and the immunoassay for the IL-6/sIL-6R complex (j) in representative sera from patients with s-JIA (4 out of 15 tested in the XG-1 assay and 4 out of 8 tested in the Hep 3b assay). Results are shown as means + SE, represented by the vertical bars, of triplicate determinations.
) and the immunoassay for the IL-6/sIL-6R complex (j) in representative sera from patients with s-JIA (4 out of 15 tested in the XG-1 assay and 4 out of 8 tested in the Hep 3b assay). Results are shown as means + SE, represented by the vertical bars, of triplicate determinations.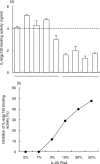
Similar articles
-
Soluble IL-6 receptor potentiates the antagonistic activity of soluble gp130 on IL-6 responses.J Immunol. 1998 Dec 1;161(11):6347-55. J Immunol. 1998. PMID: 9834125
-
Measurements of IL-6, soluble IL-6 receptor and soluble gp130 in sera of B-cell lymphoma patients. Does viscum album treatment affect these parameters?Biomed Pharmacother. 2002 May;56(3):152-8. doi: 10.1016/s0753-3322(02)00165-8. Biomed Pharmacother. 2002. PMID: 12046687 Clinical Trial.
-
Interleukin-6 signalling in juvenile idiopathic arthritis is limited by proteolytically cleaved soluble interleukin-6 receptor.Rheumatology (Oxford). 2006 Dec;45(12):1485-9. doi: 10.1093/rheumatology/kel154. Epub 2006 May 11. Rheumatology (Oxford). 2006. PMID: 16690760
-
The role of transsignalling via the agonistic soluble IL-6 receptor in human diseases.Biochim Biophys Acta. 2002 Nov 11;1592(3):323-43. doi: 10.1016/s0167-4889(02)00325-7. Biochim Biophys Acta. 2002. PMID: 12421676 Review.
-
The soluble IL-6 receptors: serum levels and biological function.Cell Mol Biol (Noisy-le-grand). 2001 Jun;47(4):583-97. Cell Mol Biol (Noisy-le-grand). 2001. PMID: 11502067 Review.
Cited by
-
Targeting interleukin-6 for noninfectious uveitis.Clin Ophthalmol. 2015 Sep 11;9:1697-702. doi: 10.2147/OPTH.S68595. eCollection 2015. Clin Ophthalmol. 2015. PMID: 26392750 Free PMC article. Review.
-
Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease.J Am Coll Cardiol. 2008 Oct 7;52(15):1201-10. doi: 10.1016/j.jacc.2008.05.060. J Am Coll Cardiol. 2008. PMID: 18926322 Free PMC article. Review.
-
Interleukin-6 in surgery, trauma, and critical care part II: clinical implications.J Intensive Care Med. 2011 Mar-Apr;26(2):73-87. doi: 10.1177/0885066610384188. J Intensive Care Med. 2011. PMID: 21464062 Free PMC article. Review.
-
Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study.Ann Rheum Dis. 2007 May;66(5):589-98. doi: 10.1136/ard.2006.061853. Epub 2006 Dec 14. Ann Rheum Dis. 2007. PMID: 17170049 Free PMC article.
-
Analytic review: Interleukin-6 in surgery, trauma, and critical care: part I: basic science.J Intensive Care Med. 2011 Jan-Feb;26(1):3-12. doi: 10.1177/0885066610395678. J Intensive Care Med. 2011. PMID: 21262749 Free PMC article. Review.
References
-
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. - PubMed
-
- Yoshizaki K, Matsuda T, Nishimoto N, et al. Pathogenic significance of interelukin-6 (IL-6/BSF-2) in Castleman's disease. Blood. 1989;74:1360–7. - PubMed
-
- Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, Shirai T, Kishimoto T, Yoshizaki K. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95:56–61. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

