Effect of intracellular pH on rotational speed of bacterial flagellar motors
- PMID: 12562788
- PMCID: PMC142873
- DOI: 10.1128/JB.185.4.1190-1194.2003
Effect of intracellular pH on rotational speed of bacterial flagellar motors
Abstract
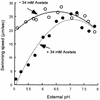
Weak acids such as acetate and benzoate, which partially collapse the transmembrane proton gradient, not only mediate pH taxis but also impair the motility of Escherichia coli and Salmonella at an external pH of 5.5. In this study, we examined in more detail the effect of weak acids on motility at various external pH values. A change of external pH over the range 5.0 to 7.8 hardly affected the swimming speed of E. coli cells in the absence of 34 mM potassium acetate. In contrast, the cells decreased their swimming speed significantly as external pH was shifted from pH 7.0 to 5.0 in the presence of 34 mM acetate. The total proton motive force of E. coli cells was not changed greatly by the presence of acetate. We measured the rotational rate of tethered E. coli cells as a function of external pH. Rotational speed decreased rapidly as the external pH was decreased, and at pH 5.0, the motor stopped completely. When the external pH was returned to 7.0, the motor restarted rotating at almost its original level, indicating that high intracellular proton (H+) concentration does not irreversibly abolish flagellar motor function. Both the swimming speeds and rotation rates of tethered cells of Salmonella also decreased considerably when the external pH was shifted from pH 7.0 to 5.5 in the presence of 20 mM benzoate. We propose that the increase in the intracellular proton concentration interferes with the release of protons from the torque-generating units, resulting in slowing or stopping of the motors.
Figures


Similar articles
-
Effect of the MotA(M206I) Mutation on Torque Generation and Stator Assembly in the Salmonella H+-Driven Flagellar Motor.J Bacteriol. 2019 Feb 25;201(6):e00727-18. doi: 10.1128/JB.00727-18. Print 2019 Mar 15. J Bacteriol. 2019. PMID: 30642987 Free PMC article.
-
Effect of intracellular pH on the torque-speed relationship of bacterial proton-driven flagellar motor.J Mol Biol. 2009 Feb 20;386(2):332-8. doi: 10.1016/j.jmb.2008.12.034. Epub 2008 Dec 24. J Mol Biol. 2009. PMID: 19133273
-
Quantitative measurements of proton motive force and motility in Bacillus subtilis.J Bacteriol. 1980 Dec;144(3):891-7. doi: 10.1128/jb.144.3.891-897.1980. J Bacteriol. 1980. PMID: 6254950 Free PMC article.
-
Constraints on models for the flagellar rotary motor.Philos Trans R Soc Lond B Biol Sci. 2000 Apr 29;355(1396):491-501. doi: 10.1098/rstb.2000.0590. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 10836502 Free PMC article. Review.
-
Torque generation by the flagellar rotary motor.Biophys J. 1995 Apr;68(4 Suppl):163S-166S; discussion 166S-167S. Biophys J. 1995. PMID: 7787060 Free PMC article. Review.
Cited by
-
Nonconventional cation-coupled flagellar motors derived from the alkaliphilic Bacillus and Paenibacillus species.Extremophiles. 2017 Jan;21(1):3-14. doi: 10.1007/s00792-016-0886-y. Epub 2016 Oct 22. Extremophiles. 2017. PMID: 27771767 Review.
-
Yersinia enterocolitica type III secretion depends on the proton motive force but not on the flagellar motor components MotA and MotB.Infect Immun. 2004 Jul;72(7):4004-9. doi: 10.1128/IAI.72.7.4004-4009.2004. Infect Immun. 2004. PMID: 15213145 Free PMC article.
-
Structural insight into the rotational switching mechanism of the bacterial flagellar motor.PLoS Biol. 2011 May;9(5):e1000616. doi: 10.1371/journal.pbio.1000616. Epub 2011 May 10. PLoS Biol. 2011. PMID: 21572987 Free PMC article.
-
The Dynamic Ion Motive Force Powering the Bacterial Flagellar Motor.Front Microbiol. 2021 Apr 13;12:659464. doi: 10.3389/fmicb.2021.659464. eCollection 2021. Front Microbiol. 2021. PMID: 33927708 Free PMC article. Review.
-
The C-terminal periplasmic domain of MotB is responsible for load-dependent control of the number of stators of the bacterial flagellar motor.Biophysics (Nagoya-shi). 2013 Dec 26;9:173-81. doi: 10.2142/biophysics.9.173. eCollection 2013. Biophysics (Nagoya-shi). 2013. PMID: 27493556 Free PMC article.
References
-
- Blair, D. F., and H. C. Berg. 1990. The MotA protein of E. coli is a proton-conducting component of the flagellar motor. Cell 60:439-449. - PubMed
-
- Blair, D. F., and H. C. Berg. 1991. Mutations in the MotA protein of Escherichia coli reveal domains critical for proton conduction. J. Mol. Biol. 221:1433-1442. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

