Overrepresentation of a gene family encoding extracytoplasmic solute receptors in Bordetella
- PMID: 12562821
- PMCID: PMC142875
- DOI: 10.1128/JB.185.4.1470-1474.2003
Overrepresentation of a gene family encoding extracytoplasmic solute receptors in Bordetella
Abstract
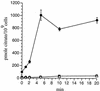
A family of genes that are likely to encode extracytoplasmic solute receptors is strongly overrepresented in several beta-proteobacteria, including Bordetella pertussis. This gene family, of which members have been called bug genes, contains some examples that are contained within polycistronic operons coding for tripartite uptake transporters of the TTT family, while the vast majority are "orphan" genes. Proteomic and functional analyses demonstrated that several of these genes are expressed in B. pertussis, and one is involved in citrate uptake. The bug genes probably form an ancient family that has been subjected to a large expansion in a restricted phylogenic group.
Figures


References
-
- Antoine, R., S. Alonzo, D. Raze, L. Coutte, S. Lesjean, E. Willery, C. Locht, and F. Jacob-Dubuisson. 2000. New virulence-activated and virulence-repressed genes identified by systematic gene inactivation and generation of transcriptional fusions in Bordetella pertussis. J. Bacteriol. 182:5902-5905. - PMC - PubMed
-
- Antoine, R., D. Raze, and C. Locht. 2000. Genomics of Bordetella pertussis toxins. Int. J. Med. Microbiol. 290:301-305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

