The actions of anandamide on rat superficial medullary dorsal horn neurons in vitro
- PMID: 12562891
- PMCID: PMC2342784
- DOI: 10.1113/jphysiol.2002.035063
The actions of anandamide on rat superficial medullary dorsal horn neurons in vitro
Abstract
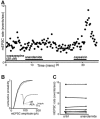
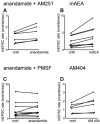
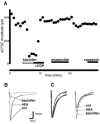
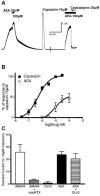
Whole-cell patch-clamp recordings were made from neurons in the trigeminal nucleus caudalis and trigeminal ganglion, in vitro, to investigate the cellular actions of the endogenous cannabinoid, anandamide. Anandamide has been shown to act through both the cannabinoid receptor 1 (CB1) and the vanilloid receptor 1 (VR1). Anandamide (30 microM) caused a 54 % increase in the rate of miniature excitatory post-synaptic currents (mEPSCs), without affecting their amplitude. The effect of anandamide was blocked by the VR1 antagonist capsazepine (20 microM), but not by the CB1-specific antagonist AM251 (3 microM). Application of the VR1 receptor agonist capsaicin (300 nM) caused a 4200 % increase in the mEPSC rate. In dissociated trigeminal ganglion neurons, both anandamide and capsaicin caused an outward current in neurons that were voltage clamped at +40 mV. The maximal outward current produced by anandamide (EC50, 10 microM) was 45 % of that produced by capsaicin (10 microM). Co-application of the VR1 antagonist capsazepine (30 microM) completely reversed the effects of both capsaicin and anandamide. The anandamide transport inhibitor, AM404 (30 microM) caused a 40 % increase in mEPSC rate in the slice preparation and an outward current in dissociated neurons. The latter current was reversed by the VR1 antagonist iodoresiniferatoxin (1 microM). The fatty acid amide hydrolase (FAAH) inhibitors phenylmethylsulfonyl fluoride (PMSF) (20 microM) and OL53 (1 microM) did not enhance the effect of anandamide in either the slice or dissociated neuron preparations. These results suggest that within the superficial medullary dorsal horn, anandamide (30 microM) acts presynaptically to enhance the release of glutamate via activation of the VR1 receptor.
Figures

Similar articles
-
Inhibition of fatty acid amide hydrolase unmasks CB1 receptor and TRPV1 channel-mediated modulation of glutamatergic synaptic transmission in midbrain periaqueductal grey.Br J Pharmacol. 2011 Jul;163(6):1214-22. doi: 10.1111/j.1476-5381.2010.01157.x. Br J Pharmacol. 2011. PMID: 21175570 Free PMC article.
-
Anandamide is a partial agonist at native vanilloid receptors in acutely isolated mouse trigeminal sensory neurons.Br J Pharmacol. 2002 Oct;137(4):421-8. doi: 10.1038/sj.bjp.0704904. Br J Pharmacol. 2002. PMID: 12359623 Free PMC article.
-
Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors.J Neurosci. 2003 Apr 15;23(8):3136-44. doi: 10.1523/JNEUROSCI.23-08-03136.2003. J Neurosci. 2003. PMID: 12716921 Free PMC article.
-
Possible mechanisms of cannabinoid-induced antinociception in the spinal cord.Eur J Pharmacol. 2001 Oct 19;429(1-3):93-100. doi: 10.1016/s0014-2999(01)01309-7. Eur J Pharmacol. 2001. PMID: 11698030 Review.
-
Anandamide: some like it hot.Trends Pharmacol Sci. 2001 Jul;22(7):346-9. doi: 10.1016/s0165-6147(00)01712-0. Trends Pharmacol Sci. 2001. PMID: 11431028 Review.
Cited by
-
Excitatory Effects of Calcitonin Gene-Related Peptide (CGRP) on Superficial Sp5C Neurons in Mouse Medullary Slices.Int J Mol Sci. 2021 Apr 6;22(7):3794. doi: 10.3390/ijms22073794. Int J Mol Sci. 2021. PMID: 33917574 Free PMC article.
-
Physiological temperatures drive glutamate release onto trigeminal superficial dorsal horn neurons.J Neurophysiol. 2014 Jun 1;111(11):2222-31. doi: 10.1152/jn.00912.2013. Epub 2014 Mar 5. J Neurophysiol. 2014. PMID: 24598529 Free PMC article.
-
New capsaicin analogs as molecular rulers to define the permissive conformation of the mouse TRPV1 ligand-binding pocket.Elife. 2020 Nov 9;9:e62039. doi: 10.7554/eLife.62039. Elife. 2020. PMID: 33164749 Free PMC article.
-
Inhibition of fatty acid amide hydrolase unmasks CB1 receptor and TRPV1 channel-mediated modulation of glutamatergic synaptic transmission in midbrain periaqueductal grey.Br J Pharmacol. 2011 Jul;163(6):1214-22. doi: 10.1111/j.1476-5381.2010.01157.x. Br J Pharmacol. 2011. PMID: 21175570 Free PMC article.
-
Attenuation of acid induced oesophagitis in VR-1 deficient mice.Gut. 2006 Jan;55(1):34-40. doi: 10.1136/gut.2005.066795. Epub 2005 Aug 9. Gut. 2006. PMID: 16091555 Free PMC article.
References
-
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–688. - PubMed
-
- Boger DL, Sato H, Lerner AE, Hedrick MP, Fecik RA, Miyauchi H, Wilkie GD, Austin BJ, Patricelli MP, Cravatt BF. Exceptionally potent inhibitors of fatty acid amide hydrolase: the enzyme responsible for degradation of endogenous oleamide and anandamide. Proc Natl Acad Sci U S A. 2000;97:5044–5049. - PMC - PubMed
-
- Calignano A. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. - PubMed
-
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor – a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

