Creatine kinase injection restores contractile function in creatine-kinase-deficient mouse skeletal muscle fibres
- PMID: 12562893
- PMCID: PMC2342641
- DOI: 10.1113/jphysiol.2002.034793
Creatine kinase injection restores contractile function in creatine-kinase-deficient mouse skeletal muscle fibres
Abstract
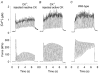
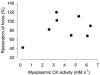
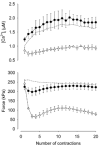
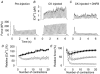
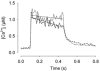
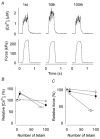
Viable genetically engineered animals generally exhibit adaptations to the altered genotype, which may mask the role of the protein of interest. We now describe a novel method by which the direct effects of the altered genotype can be distinguished from secondary adaptive changes in isolated adult skeletal muscle cells. We studied contractile function and intracellular Ca2+ handling in single skeletal muscle fibres that are completely deficient of creatine kinase (CK; CK-/-) before and after microinjection of purified CK (injected together with the fluorescent Ca2+ indicator indo-1). The mean total CK activity after CK injection was estimated to be approximately 4 mM s-1, which is approximately 5 % of the activity in wild-type muscle fibres. After CK injection, CK-/- fibres approached the wild-type phenotype in several aspects: (a) the free myoplasmic [Ca2+] ([Ca2+]i) increased and force showed little change during a period of high-intensity stimulation (duty cycle, i.e. tetanic duration divided by tetanic interval = 0.67); (b) [Ca2+]i did not decline during a brief (350 ms) tetanus; (c) during low-intensity fatiguing stimulation (duty cycle = 0.14), tetanic [Ca2+]i increased over the first 10 tetani, and thereafter it decreased; (d) tetanic [Ca2+]i and force did not display a transient reduction in the second tetanus of low-intensity fatiguing stimulation. Conversely, tetanic force in the unfatigued state was lower in CK-/- than in wild-type fibres, and this difference persisted after CK injection. Injection of inactivated CK had no obvious effect on any of the measured parameters. In conclusion, microinjection of CK into CK-/- fibres markedly restores many, but not all, aspects of the wild-type phenotype.
Figures
Similar articles
-
Mitochondrial function in intact skeletal muscle fibres of creatine kinase deficient mice.J Physiol. 2003 Oct 15;552(Pt 2):393-402. doi: 10.1113/jphysiol.2003.050732. J Physiol. 2003. PMID: 14561823 Free PMC article.
-
Role of myoplasmic phosphate in contractile function of skeletal muscle: studies on creatine kinase-deficient mice.J Physiol. 2001 Jun 1;533(Pt 2):379-88. doi: 10.1111/j.1469-7793.2001.0379a.x. J Physiol. 2001. PMID: 11389199 Free PMC article.
-
Mitochondrial and myoplasmic [Ca2+] in single fibres from mouse limb muscles during repeated tetanic contractions.J Physiol. 2003 Aug 15;551(Pt 1):179-90. doi: 10.1113/jphysiol.2003.043927. Epub 2003 Jun 18. J Physiol. 2003. PMID: 12815178 Free PMC article.
-
Mechanisms of fatigue induced by isometric contractions in exercising humans and in mouse isolated single muscle fibres.Clin Exp Pharmacol Physiol. 2009 Mar;36(3):334-9. doi: 10.1111/j.1440-1681.2008.05021.x. Epub 2008 Jul 29. Clin Exp Pharmacol Physiol. 2009. PMID: 18671711 Review.
-
Mechanisms underlying changes of tetanic [Ca2+]i and force in skeletal muscle.Acta Physiol Scand. 1996 Mar;156(3):407-16. doi: 10.1046/j.1365-201X.1996.196000.x. Acta Physiol Scand. 1996. PMID: 8729701 Review.
Cited by
-
Competing effects of activation history on force and cytosolic Ca2+ in intact single mice myofibers.Pflugers Arch. 2025 Mar;477(3):407-419. doi: 10.1007/s00424-024-03061-5. Epub 2024 Dec 30. Pflugers Arch. 2025. PMID: 39738587
-
Effect of low cytoplasmic [ATP] on excitation-contraction coupling in fast-twitch muscle fibres of the rat.J Physiol. 2004 Oct 15;560(Pt 2):451-68. doi: 10.1113/jphysiol.2004.069112. Epub 2004 Aug 12. J Physiol. 2004. PMID: 15308682 Free PMC article.
-
Molecular Basis for Exercise-Induced Fatigue: The Importance of Strictly Controlled Cellular Ca2+ Handling.Cold Spring Harb Perspect Med. 2018 Feb 1;8(2):a029710. doi: 10.1101/cshperspect.a029710. Cold Spring Harb Perspect Med. 2018. PMID: 28432118 Free PMC article. Review.
-
Limited oxygen diffusion accelerates fatigue development in mouse skeletal muscle.J Physiol. 2006 Apr 15;572(Pt 2):551-9. doi: 10.1113/jphysiol.2005.104521. Epub 2006 Feb 2. J Physiol. 2006. PMID: 16455685 Free PMC article.
-
Long-term wheel-running prevents reduction of grip strength in type 2 diabetic rats.Physiol Rep. 2021 Sep;9(18):e15046. doi: 10.14814/phy2.15046. Physiol Rep. 2021. PMID: 34558206 Free PMC article.
References
-
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol. 1995;80:497–527. - PubMed
-
- Allen DG, Lännergren J, Westerblad H. Intracellular ATP measured with luciferin/luciferase in isolated single mouse skeletal muscle fibres. Pflugers Arch. 2002;443:836–842. - PubMed
-
- Balog EM, Fruen BR, Kane PK, Louis CF. Mechanisms of Pi regulation of the skeletal muscle SR Ca2+ release channel. Am J Physiol Cell Physiol. 2000;278:C601–611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

