Protein kinase C is necessary for recovery from the thyrotropin-releasing hormone-induced r-ERG current reduction in GH3 rat anterior pituitary cells
- PMID: 12562894
- PMCID: PMC2342738
- DOI: 10.1113/jphysiol.2002.034611
Protein kinase C is necessary for recovery from the thyrotropin-releasing hormone-induced r-ERG current reduction in GH3 rat anterior pituitary cells
Abstract
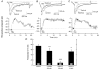
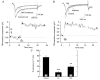
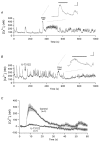
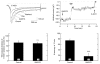
The biochemical cascade linking activation of phospholipase C-coupled thyrotropin-releasing hormone (TRH) receptors to rat ERG (r-ERG) channel modulation was studied in situ using perforated-patch clamped adenohypophysial GH3 cells and pharmacological inhibitors. To check the recent suggestion that Rho kinase is involved in the TRH-induced r-ERG current suppression, the hormonal effects were studied in cells pretreated with the Rho kinase inhibitors Y-27632 and HA-1077. The TRH-induced r-ERG inhibition was not significantly modified in the presence of the inhibitors. Surprisingly, the hormonal effects became irreversible in the presence of HA-1077 but not in the presence of the more potent Rho kinase inhibitor Y-27632. Further experiments indicated that the effect of HA-1077 correlated with its ability to inhibit protein kinase C (PKC). The hormonal effects also became irreversible in cells in which PKC activity was selectively impaired with GF109203X, Gö6976 or long-term incubation with phorbol esters. Furthermore, the reversal of the effects of TRH, but not its ability to suppress r-ERG currents, was blocked if diacylglycerol generation was prevented by blocking phospholipase C activity with U-73122. Our results suggest that a pathway involving an as yet unidentified protein kinase is the main cause of r-ERG inhibition in perforated-patch clamped GH3 cells. Furthermore, they demonstrate that although not necessary to trigger the ERG current reductions induced by TRH, an intracellular signal cascade involving phosphatidylinositol-4,5-bisphosphate hydrolysis by phospholipase C, activation of an alpha/betaII conventional PKC and one or more dephosphorylation steps catalysed by protein phosphatase 2A, mediates recovery of ERG currents following TRH withdrawal.
Figures




References
-
- Akita Y, Ohno S, Yajima Y, Konno Y, Saido TC, Mizuno K, Chida K, Osada S, Kuroki T, Kawashima S, Suzuki K. J Biol Chem. 1994;269:4653–4660. - PubMed
-
- Arcangeli A, Rosati B, Cherubini A, Crociani O, Fontana L, Ziller C, Wanke E, Olivotto M. HERG- and IRK-like inward rectifier currents are sequentially expressed during neuronal development of neural crest cells and their derivatives. Eur J Neurosci. 1997;9:2596–2604. - PubMed
-
- Barros F, Del Camino D, Pardo LA, Palomero T, Giráldez T, de la Peña P. Demonstration of an inwardly rectifying K+ current component modulated by thyrotropin-releasing hormone and caffeine in GH3 rat anterior pituitary cells. Pflugers Arch. 1997;435:119–129. - PubMed
-
- Barros F, Delgado LM, Del Camino D, de la Peña P. Characteristics and modulation by thyrotropin-releasing hormone of an inwardly rectifying K+ current in patch-perforated GH3 anterior pituitary cells. Pflugers Arch. 1992;422:31–39. - PubMed
-
- Barros F, Delgado LM, Maciá C, de la Peña P. Effects of hypothalamic peptides on electrical activity and membrane currents of ‘patch perforated’ clamped GH3 anterior pituitary cells. FEBS Lett. 1991;279:33–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

