Polarized distribution of HCO3- transport in human normal and cystic fibrosis nasal epithelia
- PMID: 12562898
- PMCID: PMC2342788
- DOI: 10.1113/jphysiol.2002.034447
Polarized distribution of HCO3- transport in human normal and cystic fibrosis nasal epithelia
Abstract
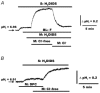
The polarized distribution of HCO3- transport was investigated in human nasal epithelial cells from normal and cystic fibrosis (CF) tissues. To test for HCO3- transport via conductive versus electroneutral Cl-/HCO3- exchange (anion exchange, AE) pathways, nasal cells were loaded with the pH probe 2',7'-bis(carboxyethyl)-5(6)-carboxyfluorescein and mounted in a bilateral perfusion chamber. In normal, but not CF, epithelia, replacing mucosal Cl- with gluconate caused intracellular pH (pHi) to increase, and the initial rates (Delta pH min-1) of this increase were modestly augmented (approximately 26 %) when normal cells were pretreated with forskolin (10 microM). Recovery from this alkaline shift was dependent on mucosal Cl-, was insensitive to the AE inhibitor 4,4'-diisothiocyanatodihydrostilbene-2,2'-disulfonic acid (H2DIDS; 1.5 mM), but was sensitive to the cystic fibrosis transmembrane conductance regulator (CFTR) channel inhibitor diphenylamine-2-carboxylate (DPC; 100 microM). In contrast, removal of serosal Cl- caused pHi to alkalinize in both normal and CF epithelia. Recovery from this alkaline challenge was dependent on serosal Cl- and blocked by H2DIDS. Additional studies showed that serosally applied Ba2+ (5.0 mM) in normal, but not CF, cells induced influx of HCO3- across the apical membrane that was reversibly blocked by mucosal DPC. In a final series of studies, normal and CF cells acutely alkaline loaded by replacing bilateral Krebs bicarbonate Ringer (KBR) with Hepes-buffered Ringer solution exhibited basolateral, but not apical, recovery from an alkaline challenge that was dependent on Cl-, independent of Na+ and blocked by H2DIDS. We conclude that: (1) normal, but not CF, nasal epithelia have a constitutively active DPC-sensitive HCO3- influx/efflux pathway across the apical membrane of cells, consistent with the movement of HCO3- via CFTR; and (2) both normal and CF nasal epithelia have Na+-independent, H2DIDS-sensitive AE at their basolateral domain.
Figures
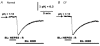
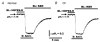

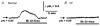


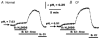


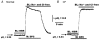
References
-
- Anderson MP, Gregory RJ, Thompson S, Souza DW, Paul S, Mulligan RC, Smith AE, Welsh MJ. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–205. - PubMed
-
- Boron WF. Intracellular pH regulation in epithelial cells. Annu Rev Physiol. 1986;48:377–388. - PubMed
-
- Clarke LL, Boucher RC. Chloride secretory response to extracellular ATP in normal and cystic fibrosis nasal epithelia. Am J Physiol. 1992;263:C348–356. - PubMed
-
- Coakley RD, Paradiso AM, Grubb BR, Gatzy JT, Chadburn JL, Boucher RC. Abnormal airway surface liquid pH (pHASL) regulation in cultured CF bronchial epithelium. Pediatr Pulmonol Suppl. 2000;14:194.
-
- Devor DC, Frizzell RA. Modulation of K+ channels by arachidonic acid in T84 cells. II. Activation of a Ca2+-independent K+ channel. Am J Physiol. 1998;274:C149–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

