Role of the transverse-axial tubule system in generating calcium sparks and calcium transients in rat atrial myocytes
- PMID: 12562899
- PMCID: PMC2342655
- DOI: 10.1113/jphysiol.2002.034355
Role of the transverse-axial tubule system in generating calcium sparks and calcium transients in rat atrial myocytes
Abstract
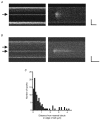
Cardiac atrial cells lack a regular system of transverse tubules like that in cardiac ventricular cells. Nevertheless, many atrial cells do possess an irregular internal transverse-axial tubular system (TATS). To investigate the possible role of the TATS in excitation-contraction coupling in atrial myocytes, we visualized the TATS (labelled with the fluorescent indicator, Di-8-ANEPPS) simultaneously with Ca2+ transients and/or Ca2+ sparks (fluo-4). In confocal transverse linescan images of field-stimulated cells, whole-cell Ca2+ transients had two morphologies: 'U-shaped' transients and irregular or 'W-shaped' transients with a varying number of points of origin of the Ca2+ transient. About half (54 %, n =289 cells, 13 animals) of the cells had a TATS. Cells with TATS had a larger mean diameter (13.2 +/- 2.8 microm) than cells without TATS (11.7 +/- 2.0 microm) and were more common in the left atrium (n = 206 cells; left atrium: 76 with TATS, 30 without TATS; right atrium: 42 with TATS, 58 without TATS). Simultaneous measurement of Ca2+ sparks and sarcolemmal structures showed that cells without TATS had U-shaped transients that started at the cell periphery, and cells with TATS had W-shaped transients that began simultaneously at the cell periphery and the TATS. Most (82 out of 102 from 31 cells) 'spontaneous' (non-depolarized) Ca2+ sparks occurred within 1 microm of a sarcolemmal structure (cell periphery or TATS), and 33 % occurred within 1 pixel (0.125 microm). We conclude that the presence of a sarcolemmal membrane either at the cell periphery or in the TATS in close apposition to the sarcoplasmic reticulum is required for the initiation of an evoked Ca2+ transient and for spontaneous Ca2+ sparks.
Figures



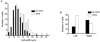
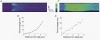
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

