Multiple conductance states of single Ca2+-activated Cl- channels in rabbit pulmonary artery smooth muscle cells
- PMID: 12562904
- PMCID: PMC2342635
- DOI: 10.1113/jphysiol.2002.033688
Multiple conductance states of single Ca2+-activated Cl- channels in rabbit pulmonary artery smooth muscle cells
Abstract
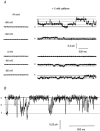
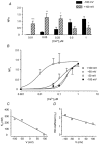
Ca2+-activated Cl- channels contribute to agonist-evoked contraction and spontaneous activity in some smooth muscle preparations. Patch pipette techniques were used to study the properties of single Ca2+-activated Cl- channels in freshly dispersed rabbit pulmonary artery myocytes. In the cell-attached recording mode, two conductance states of 3.5 and 1.8 pS were recorded either spontaneously or in response to increasing [Ca2+]i. With inside-out patches, the 3.5 pS channel current predominated at 50 nM [Ca2+]i, but at 500 nM [Ca2+]i most channels opened to the 1.8 pS level and an additional 1.2 pS channel conductance was resolved. At 1 microM [Ca2+]i all of the Cl- channels opened either to the 1.8 pS or 1.2 pS level. In 0 [Ca2+]i, no channel activity was observed at -100 mV to +100 mV, but with 10-250 nM [Ca2+]i the total single channel open probability (NP(o)) increased with depolarisation. This voltage dependence was not seen at higher values of [Ca2+]i. The plot of NPo vs. [Ca2+]i yielded Ca2+ affinity constants of 8 and 250 nM and Hill slopes of 1.3 and 2.3 at +100 and -100 mV, respectively. The distribution of open times was fitted by two exponentials of about 5 and 30 ms, which were neither voltage nor Ca2+ dependent. Replacement of external Cl- by I- shifted the reversal potential by about -30 mV and lengthened the longer of the two mean open times without significant effects on other kinetic parameters. Based on these data, a model for the activation of Ca2+-activated Cl- channels is proposed.
Figures







References
-
- Collier ML, Levesque PC, Kenyon JL, Hume JR. Unitary Cl− channels activated by cytoplasmic Ca2+ in canine ventricular myocytes. Circ Res. 1996;78:936–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

