Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon
- PMID: 12562910
- PMCID: PMC2342639
- DOI: 10.1113/jphysiol.2002.035147
Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon
Abstract
The electrical and synaptic properties of myenteric neurones in normal and inflamed guinea-pig distal colons were evaluated by intracellular microelectrode recording. Chronic inflammation was established 6 days following administration of trinitrobenzene sulfonic acid (TNBS). In S neurones, inflammation only altered synaptic inputs as the amplitude of fast excitatory postsynaptic potentials were significantly larger (31 +/- 2 mV compared to 20 +/- 1 mV) and they were more likely to receive slow excitatory synaptic input (85% compared to 55%). AH neurones displayed altered electrical properties in colitis compared to control tissues: they generated more action potentials during a maximal depolarising current pulse (7 +/- 1 compared to 1.6 +/- 0.2); they had a smaller after hyperpolarisation (9 +/- 2 mV s compared to 20 +/- 2 mV s); and they were more likely to receive fast excitatory synaptic input (74% compared to 17%), possess spontaneous activity (46% compared to 3%), and generate anodal break action potentials (58% compared to 19%). Although the resting membrane potential, input resistance and action potential characteristics were unaltered in AH neurones from inflamed tissues, they exhibited an enhanced Cs+-sensitive rectification of the current-voltage relationship. This suggests that the increase in excitability of AH neurones may involve a colitis-induced augmentation of the hyperpolarisation-activated cation current (Ih) in these cells. An increased excitability, selectively in AH neurones, suggests that the afferent limb of intrinsic motor reflexes is disrupted in the inflamed colon and this may contribute to dysmotility associated with inflammatory diseases.
Figures
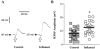
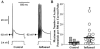
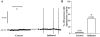
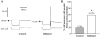


Similar articles
-
Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig.J Physiol. 2005 May 1;564(Pt 3):863-75. doi: 10.1113/jphysiol.2005.084285. Epub 2005 Mar 17. J Physiol. 2005. PMID: 15774518 Free PMC article.
-
Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon.J Physiol. 2004 May 15;557(Pt 1):191-205. doi: 10.1113/jphysiol.2004.062174. Epub 2004 Mar 12. J Physiol. 2004. PMID: 15020692 Free PMC article.
-
Changes in colonic motility and the electrophysiological properties of myenteric neurons persist following recovery from trinitrobenzene sulfonic acid colitis in the guinea pig.Neurogastroenterol Motil. 2007 Dec;19(12):990-1000. doi: 10.1111/j.1365-2982.2007.00986.x. Epub 2007 Aug 17. Neurogastroenterol Motil. 2007. PMID: 17973636
-
Pro-epileptic changes in synaptic function can be accompanied by pro-epileptic changes in neuronal excitability.Trends Neurosci. 1998 Apr;21(4):167-74. doi: 10.1016/s0166-2236(97)01182-x. Trends Neurosci. 1998. PMID: 9554727 Review.
-
Controlling the excitability of IPANs: a possible route to therapeutics.Curr Opin Pharmacol. 2002 Dec;2(6):657-64. doi: 10.1016/s1471-4892(02)00222-9. Curr Opin Pharmacol. 2002. PMID: 12482727 Review.
Cited by
-
Hyperpolarization-activated cation currents in medium-size dorsal root ganglion cells are involved in overactive bladder syndrome in rats.BMC Urol. 2020 Sep 2;20(1):140. doi: 10.1186/s12894-020-00698-z. BMC Urol. 2020. PMID: 32878607 Free PMC article.
-
Synaptic facilitation and enhanced neuronal excitability in the submucosal plexus during experimental colitis in guinea-pig.J Physiol. 2005 May 1;564(Pt 3):863-75. doi: 10.1113/jphysiol.2005.084285. Epub 2005 Mar 17. J Physiol. 2005. PMID: 15774518 Free PMC article.
-
Enteric neuroplasticity and dysmotility in inflammatory disease: key players and possible therapeutic targets.Am J Physiol Gastrointest Liver Physiol. 2019 Dec 1;317(6):G853-G861. doi: 10.1152/ajpgi.00206.2019. Epub 2019 Oct 11. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31604034 Free PMC article. Review.
-
The distribution of PKC isoforms in enteric neurons, muscle and interstitial cells of the human intestine.Histochem Cell Biol. 2006 Nov;126(5):537-48. doi: 10.1007/s00418-006-0190-5. Epub 2006 May 30. Histochem Cell Biol. 2006. PMID: 16733665
-
Neuronal regulation of the gut immune system and neuromodulation for treating inflammatory bowel disease.FASEB Bioadv. 2021 Aug 27;3(11):953-966. doi: 10.1096/fba.2021-00070. eCollection 2021 Nov. FASEB Bioadv. 2021. PMID: 34761177 Free PMC article. Review.
References
-
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am J Physiol. 1997;273:G422–435. - PubMed
-
- Bornstein JC, Furness JB, Kunze WA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? J Auton Nerv Syst. 1994;48:1–15. - PubMed
-
- Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. - PubMed
-
- Castro GA. Intestinal physiology in the parasitized host: integration, disintegration, and reconstruction of systems. Ann N Y Acad Sci. 1992;664:369–379. - PubMed
-
- Collins SM, Hurst SM, Main C, Stanley E, Khan I, Blennerhassett P, Swain M. Effect of inflammation of enteric nerves: Cytokine-induced changes in neurotransmitter content and release. Ann NY Acad Sci. 1992;664:415–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

