Molecular basis of the effect of potassium on heterologously expressed pacemaker (HCN) channels
- PMID: 12562911
- PMCID: PMC2342664
- DOI: 10.1113/jphysiol.2003.039768
Molecular basis of the effect of potassium on heterologously expressed pacemaker (HCN) channels
Abstract
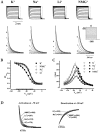
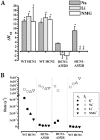
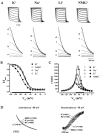
Hyperpolarization-activated cyclic-nucleotide-gated (HCN) channels modulate the firing rates of neuronal and cardiac pacemaker cells. HCN channels resemble voltage-gated K+ channels structurally, but much less is known about their structure-function correlation. Although modulation of K+ channel gating by external K+ is a well-known phenomenon, such a link has not been established for HCN channels. Here we examined the effects of external permeant (K+, Na+ and Li+) and non-permeant (NMG+) ions on HCN1 and HCN2 gating. Substituting 64 of 96 mM external K+ with Na+, Li+ or NMG+ positively shifted steady-state activation (approximately 13 mV), and preferentially slowed activation of HCN1. Mutating the pore variant C-terminal to the GYG motif in HCN1, A352, to the analogous conserved Asp in K+ channels or Arg in HCN2 produced a significant hyperpolarizing activation shift (by 5-15 mV), slowed gating kinetics (up to 6-fold), and abolished or attenuated gating responses to external K+. Whereas Na+, Li+ and NMG+ substitutions produced depolarizing activation shifts of HCN2 similar to those of HCN1, deactivation but not activation of HCN2 was exclusively decelerated. We conclude that gating and permeation of HCN channels are coupled, and that modulation of this 'pore-to-gate' coupling by external K+ is isoform-specific.
Figures

References
-
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. - PubMed
-
- Gauss R, Seifert R, Kaupp UB. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature. 1998;393:583–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

