Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing
- PMID: 12562915
- PMCID: PMC2342721
- DOI: 10.1113/jphysiol.2002.038117
Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing
Abstract
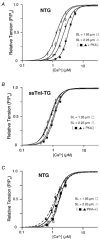
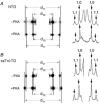
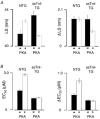
Cyclic AMP-dependent protein kinase (PKA) targets contractile proteins, troponin-I (TnI) and myosin binding protein C (MyBP-C) in the heart and induces a decrease in myofilament Ca2+ sensitivity. Yet, the effect of sarcomere length (SL) change on Ca2+ sensitivity (length-dependent activation: LDA) following PKA-dependent phosphorylation is not clear. To clarify the role of PKA-dependent phosphorylation of TnI and MyBP-C on LDA in the heart, we examined LDA in skinned myocytes from a non-transgenic (NTG) and a transgenic murine model in which the native cardiac isoform (cTnI) was completely replaced by the slow skeletal isoform of TnI (ssTnI-TG) lacking the phosphorylation sites for PKA, while retaining PKA sites on MyBP-C. In NTG myocytes, PKA treatment decreased Ca2+ sensitivity at each SL, but enhanced the impact of SL change on Ca2+ sensitivity. Despite a greater sensitivity to Ca2+ and a reduction in LDA, neither Ca2+ responsiveness nor LDA was affected by PKA treatment in ssTnI-TG myocytes. To determine whether the above observations could be explained by the lateral separation between thick and thin filaments, as suggested by others, we measured interfilament spacing by X-ray diffraction as a function of SL in skinned cardiac trabeculae in the passive state from both NTG and ssTnI-TG models before and following treatment with PKA. Phosphorylation by PKA increased lattice spacing at every SL in NTG trabeculae. However, the relationship between SL and myofilament lattice spacing in ssTnI-TG was markedly shifted downward to an overall decreased myofilament lattice spacing following PKA treatment. We conclude: (1) PKA-dependent phosphorylation enhances length-dependent activation in NTG hearts; (2) replacement of native TnI with ssTnI increases Ca2+ sensitivity of tension but reduces length-dependent activation; (3) MyBP-C phosphorylation by PKA does not alter calcium responsiveness and induces a decrease in myofilament lattice spacing at all sarcomere lengths and (4) length-dependent activation in the heart cannot be entirely explained by alterations in myofilament lattice spacing.
Figures
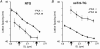
References
-
- Bremel RD, Weber A. Cooperation with actin filament in vertebrate skeletal muscle. Nat New Biol. 1972;238:97–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

