An inwardly rectifying K+ channel in bovine parotid acinar cells: possible involvement of Kir2.1
- PMID: 12562923
- PMCID: PMC2342607
- DOI: 10.1113/jphysiol.2002.035857
An inwardly rectifying K+ channel in bovine parotid acinar cells: possible involvement of Kir2.1
Erratum in
- J Physiol. 2003 Mar 15;547(Pt 3):995
Abstract
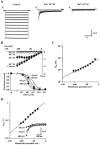
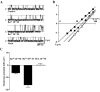
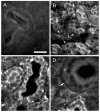
Using electrophysiological and molecular techniques, we investigated the molecular nature of an inwardly rectifying K+ channel in bovine parotid acinar (BPA) cells and examined its role in setting resting membrane potential. In whole-cell recordings from freshly isolated BPA cells, a predominant current was a K+ current rectified strongly in the inward direction. An inward conductance of the inwardly rectifying K+ (Kir) current was proportional to [K+]o(0.57). The selectivity sequence based on permeability ratios was K+ (1.00) > Rb+ (0.63) >> Li+ (0.04) = Na+ (0.02) and the sequence based on conductance ratios was K+ (1.00) >> Rb+ (0.03) = Li+ (0.03) = Na+ (0.02). The current was blocked by extracellular Ba2+ and Cs+ in a voltage- and a concentration-dependent manner, with a Kd at 0 mV of 11.6 microM and 121 mM, respectively. Cell-attached patch measurements identified 27 pS K+ channels as being the most likely to mediate whole-cell Kir currents. Addition of Ba2+ (100 microM) to the bathing solution reversibly depolarized the resting membrane potential in intact unstimulated cells. RT-PCR of RNA from bovine parotid cells revealed transcripts of bovine Kir2.1 (bKir2.1). HEK293 cells stably expressing bKir2.1 cloned from bovine parotid exhibited whole-cell and single channel Kir currents, of which electrophysiological characteristics were quantitatively similar to those of native Kir currents. Immunohistochemical studies showed a bKir2.1 immunoreactivity in BPA cells. Collectively, these results suggest that Kir2.1 may mediate native Kir currents responsible for setting resting membrane potential in BPA cells and might be, at least in part, involved in spontaneous secretion in ruminant parotid glands.
Figures




Similar articles
-
Electrophysiological characterization of native Na+-HCO3- cotransporter current in bovine parotid acinar cells.J Physiol. 2005 Oct 1;568(Pt 1):181-97. doi: 10.1113/jphysiol.2005.088633. Epub 2005 Jul 21. J Physiol. 2005. PMID: 16037094 Free PMC article.
-
Characterization of an inwardly rectifying potassium channel in the rabbit superior lacrimal gland.Invest Ophthalmol Vis Sci. 1998 Feb;39(2):308-14. Invest Ophthalmol Vis Sci. 1998. PMID: 9477987
-
Molecular cloning and expression of an inwardly rectifying K(+) channel from bovine corneal endothelial cells.Invest Ophthalmol Vis Sci. 2000 Sep;41(10):2936-44. Invest Ophthalmol Vis Sci. 2000. PMID: 10967048
-
Molecular background of leak K+ currents: two-pore domain potassium channels.Physiol Rev. 2010 Apr;90(2):559-605. doi: 10.1152/physrev.00029.2009. Physiol Rev. 2010. PMID: 20393194 Review.
-
Inwardly rectifying potassium channels.Curr Opin Cell Biol. 1999 Aug;11(4):503-8. doi: 10.1016/S0955-0674(99)80073-8. Curr Opin Cell Biol. 1999. PMID: 10449331 Review.
Cited by
-
Low-affinity spermine block mediating outward currents through Kir2.1 and Kir2.2 inward rectifier potassium channels.J Physiol. 2007 Sep 15;583(Pt 3):891-908. doi: 10.1113/jphysiol.2007.136028. Epub 2007 Jul 19. J Physiol. 2007. PMID: 17640933 Free PMC article.
-
Inward rectifier potassium (Kir) channels mediate salivary gland function and blood feeding in the lone star tick, Amblyomma americanum.PLoS Negl Trop Dis. 2019 Feb 7;13(2):e0007153. doi: 10.1371/journal.pntd.0007153. eCollection 2019 Feb. PLoS Negl Trop Dis. 2019. PMID: 30730880 Free PMC article.
-
Electrophysiological and molecular characterization of the inward rectifier in juxtaglomerular cells from rat kidney.J Physiol. 2004 Oct 15;560(Pt 2):365-76. doi: 10.1113/jphysiol.2004.070359. Epub 2004 Jul 29. J Physiol. 2004. PMID: 15284349 Free PMC article.
-
Electrophysiological characterization of native Na+-HCO3- cotransporter current in bovine parotid acinar cells.J Physiol. 2005 Oct 1;568(Pt 1):181-97. doi: 10.1113/jphysiol.2005.088633. Epub 2005 Jul 21. J Physiol. 2005. PMID: 16037094 Free PMC article.
-
BPA Directly Decreases GnRH Neuronal Activity via Noncanonical Pathway.Endocrinology. 2016 May;157(5):1980-90. doi: 10.1210/en.2015-1924. Epub 2016 Mar 2. Endocrinology. 2016. PMID: 26934298 Free PMC article.
References
-
- Barry PH, Lynch JW. Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol. 1991;121:101–117. - PubMed
-
- Church DC. Salivary function and production. In: Church DC, editor. The Ruminant Animal. Digestive Physiology and Nutrition. Englewood Cliffs, NJ, USA: Prentice Hall; 1988. pp. 117–124.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

