Amyloid beta(1-42) peptide alters the gating of human and mouse alpha-bungarotoxin-sensitive nicotinic receptors
- PMID: 12562926
- PMCID: PMC2342606
- DOI: 10.1113/jphysiol.2002.035436
Amyloid beta(1-42) peptide alters the gating of human and mouse alpha-bungarotoxin-sensitive nicotinic receptors
Abstract
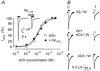
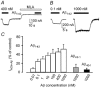
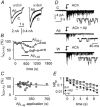
The beta-amyloid(1-42) peptide (Abeta(1-42)), a major constituent of the Alzheimer's disease amyloid plaque, specifically binds to the neuronal alpha-bungarotoxin (alpha-BuTx)-sensitive alpha7 nicotinic acetylcholine receptor (alpha7 nAChR). Accordingly, Abeta1-42 interferes with the function of alpha7 nAChRs in chick and rodent neurons. To gain insights into the human disease, we studied the action of Abeta(1-42) on human alpha7 nAChRs expressed in Xenopus oocytes. In voltage-clamped oocytes expressing the wild-type receptor, Abeta(1-42) blocked ACh-evoked currents. The block was non-competitive, required over 100 s to develop and was partially reversible. In oocytes expressing the mutant L248T receptor, Abeta(1-42) activated methyllycaconitine-sensitive currents in a dose-dependent manner. Peptide-evoked unitary events, recorded in outside-out patches, showed single-channel conductances and open duration comparable to ACh-evoked events. Abeta(1-42) had no effect on the currents evoked by glutamate, GABA or glycine in oocytes expressing human or mouse receptors for these transmitters. Muscle nAChRs are also alpha-BuTx-sensitive and we therefore investigated whether they respond to Abeta(1-42). In human kidney BOSC 23 cells expressing the fetal or adult mouse muscle nAChRs, Abeta(1-42) blocked ACh-evoked whole-cell currents, accelerating their decay. Outside-out single-channel recordings showed that the block was due to a reduced channel open probability and enhanced block upon ACh application. We also report that the inverse peptide Abeta(42-1), but not Abeta(40-1), partially mimicked the effects of the physiological Abeta(1-42) peptide. Possible implications for degenerative neuronal and muscular diseases are discussed.
Figures

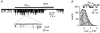
Similar articles
-
Beta-amyloid peptide activates non-alpha7 nicotinic acetylcholine receptors in rat basal forebrain neurons.J Neurophysiol. 2003 Nov;90(5):3130-6. doi: 10.1152/jn.00616.2003. Epub 2003 Jul 30. J Neurophysiol. 2003. PMID: 12890800
-
Transgenic mice over-expressing human beta-amyloid have functional nicotinic alpha 7 receptors.Neuroscience. 2006 Feb;137(3):795-805. doi: 10.1016/j.neuroscience.2005.10.007. Epub 2005 Nov 21. Neuroscience. 2006. PMID: 16303255
-
beta-Amyloid activates presynaptic alpha7 nicotinic acetylcholine receptors reconstituted into a model nerve cell system: involvement of lipid rafts.Eur J Neurosci. 2010 Mar;31(5):788-96. doi: 10.1111/j.1460-9568.2010.07116.x. Eur J Neurosci. 2010. PMID: 20374280
-
Nicotinic receptor agonists and antagonists increase sAPPalpha secretion and decrease Abeta levels in vitro.Neurochem Int. 2009 Mar-Apr;54(3-4):237-44. doi: 10.1016/j.neuint.2008.12.001. Epub 2008 Dec 7. Neurochem Int. 2009. PMID: 19111588
-
Acetylcholine receptor/channel molecules of insects.EXS. 1993;63:81-97. doi: 10.1007/978-3-0348-7265-2_5. EXS. 1993. PMID: 7678532 Review.
Cited by
-
Rescue of amyloid-Beta-induced inhibition of nicotinic acetylcholine receptors by a peptide homologous to the nicotine binding domain of the alpha 7 subtype.PLoS One. 2013 Jul 22;8(7):e67194. doi: 10.1371/journal.pone.0067194. Print 2013. PLoS One. 2013. PMID: 23894286 Free PMC article.
-
A cholinergic trigger drives learning-induced plasticity at hippocampal synapses.Nat Commun. 2013;4:2760. doi: 10.1038/ncomms3760. Nat Commun. 2013. PMID: 24217681 Free PMC article.
-
Cholinergic receptors: functional role of nicotinic ACh receptors in brain circuits and disease.Pflugers Arch. 2013 Apr;465(4):441-50. doi: 10.1007/s00424-012-1200-1. Epub 2013 Jan 11. Pflugers Arch. 2013. PMID: 23307081 Free PMC article. Review.
-
Neuronal and Glial α7 Nicotinic Acetylcholine Receptors: Role in Alzheimer's Disease Pathophysiology.Life (Basel). 2025 Jun 28;15(7):1032. doi: 10.3390/life15071032. Life (Basel). 2025. PMID: 40724534 Free PMC article. Review.
-
Amyloid-β peptide: Dr. Jekyll or Mr. Hyde?J Alzheimers Dis. 2013;33 Suppl 1(0 1):S111-20. doi: 10.3233/JAD-2012-129033. J Alzheimers Dis. 2013. PMID: 22735675 Free PMC article. Review.
References
-
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nature Neurosci. 1998;1:602–609. - PubMed
-
- Coles M, Bicknell W, Watson AA, Fairlie DP, Craik DJ. Solution structure of amyloid β-peptide(1–40) in a water-micelle environment). Is the membrane spanning domain where we think it is? Biochemistry. 1998;37:11064–11077. - PubMed
-
- Dineley KT, Bell K, Bui D, Sweatt JD. β-Amyloid peptide activates α7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem. 2002;277:25056–25061. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

