Effects of oxidation and cytosolic redox conditions on excitation-contraction coupling in rat skeletal muscle
- PMID: 12562929
- PMCID: PMC2342741
- DOI: 10.1113/jphysiol.2002.035204
Effects of oxidation and cytosolic redox conditions on excitation-contraction coupling in rat skeletal muscle
Abstract
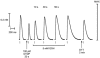
In this study the effects of oxidation and reduction on various steps in the excitation-contraction (E-C) coupling sequence was examined in mammalian skeletal muscle. In mechanically skinned fast-twitch fibres, electric field stimulation was used to generate action potentials in the sealed transverse-tubular (T-) system, thereby eliciting twitch responses, which are a sensitive measure of Ca2+ release. Treatment of fibres with the oxidant H2O2 (200 microM and 10 mM) for 2-5 min markedly potentiated caffeine-induced Ca2+ release and the force response to partial depolarisation of the T-system (by solution substitution). Importantly, such H2O2 treatment had no effect at all on any aspect of the twitch response (peak amplitude, rate of rise, decay rate constant and half-width), except in cases where it interfered with the T-system potential or voltage-sensor activation, resulting in a reduction or abolition of the twitch response. Exposure to strong thiol reductants, dithiothreitol (DTT, 10 mM) and reduced glutathione (GSH, 5 mM), did not affect the twitch response over 5 min, nor did varying the glutathione ratio (reduced to oxidised glutathione) from the level present endogenously in the cytosol of a rested fibre (30:1) to the comparatively oxidised level of 3:1. In fibres that had been oxidised by H2O2 (10 mM) (or by 2,2'-dithiodipyridine, 100 microM), exposure to GSH (5 mM) caused potentiation of twitch force (by approximately 20 % for H2O2); this effect was due to the increase in the Ca2+ sensitivity of the contractile apparatus that occurs under such circumstances and was fully reversed by subsequent exposure to 10 mM DTT. We conclude that: (a) the redox potential across the sarcomplamsic reticulum has no noticeable direct effect on normal E-C coupling in mammalian skeletal muscle, (b) oxidising the Ca2+-release channels and greatly increasing their sensitivity to Ca2+-induced Ca2+ release does not alter the amount of Ca2+ released by an action potential and (c) oxidation potentiates twitches by a GSH-mediated increase in the Ca2+ sensitivity of the contractile apparatus.
Figures


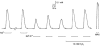



References
-
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol. 1995;80:497–527. - PubMed
-
- Allen DG, Lännergren J, Westerblad H. Intracellular ATP measured with luciferin/luciferase in isolated single mouse skeletal muscle fibres. Pflügers Arch. 2002;443:836–842. - PubMed
-
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1, 4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. J Muscle Res Cell Motil. 1996;17:55–67. - PubMed
-
- Balog EM, Fitts RH. Effects of depolarisation and low intercellular pH on charge movement currents of frog skeletal muscle fibers. J Appl Physiol. 2001;90:228–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

