A mechanism underlying the sexually dimorphic ACTH response to lipopolysaccharide in rats: sex steroid modulation of cytokine binding sites in the hypothalamus
- PMID: 12562959
- PMCID: PMC2342603
- DOI: 10.1113/jphysiol.2002.032169
A mechanism underlying the sexually dimorphic ACTH response to lipopolysaccharide in rats: sex steroid modulation of cytokine binding sites in the hypothalamus
Abstract
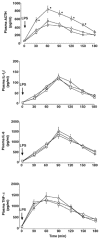
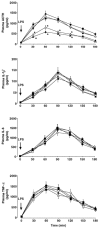
It is well established that the hypothalamic-pituitary-adrenal responses to immune stressors are sexually dimorphic in rodents (females > males), but the underlying mechanism is still unclear. To investigate the mechanism, in this study we examined whether the sex steroid environment affects the following variables in male and female rats: (1) plasma levels of ACTH, interleukin (IL)-1beta, IL-6 and tumour necrosis factor-alpha (TNF-alpha) after systemic lipopolysaccharide (LPS) administration; (2) static concentrations of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) in the mediobasal hypothalamus (MBH) and those of ACTH in the anterior pituitary (AP); and (3) the binding characteristics of IL-1beta, IL-6 and TNF-alpha in the MBH and AP. LPS-induced ACTH release was significantly higher in female than in male rats, and this sexual difference was abolished by performing gonadectomy in both sexes. Administration of physiological doses of testosterone and oestradiol to gonadectomized males and females, respectively, restored the altered ACTH responses to normal. Changes in the sex steroid milieu did not affect plasma cytokine responses to LPS, tissue contents of CRH, AVP and ACTH, or the IL-6 binding characteristics in the MBH and AP. However, the number of IL-1beta and TNF-alpha binding sites, but not their binding affinities, in the MBH showed significant changes according to altered sex hormone milieu, in the same direction as the LPS-induced ACTH response. These results suggest that the hypothalamic sensitivity to peripheral IL-1beta and TNF-alpha may be an important mechanism underlying the sexually dimorphic ACTH response to LPS in rats.
Figures




Similar articles
-
Influence of nitric oxide synthase inhibitors on the ACTH and cytokine responses to peripheral immune signals.J Neuroendocrinol. 1998 May;10(5):353-62. J Neuroendocrinol. 1998. PMID: 9663649
-
Temporal profiles of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in the plasma and hypothalamic paraventricular nucleus after intravenous or intraperitoneal administration of lipopolysaccharide in the rat: estimation by push-pull perfusion.Endocr J. 1999 Aug;46(4):487-96. doi: 10.1507/endocrj.46.487. Endocr J. 1999. PMID: 10580740
-
Modulatory role of the epinergic system in the neuroendocrine-immune system function.Neuroimmunomodulation. 2000;8(2):98-106. doi: 10.1159/000026459. Neuroimmunomodulation. 2000. PMID: 10965235
-
Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis.J Endotoxin Res. 2003;9(1):3-24. doi: 10.1179/096805103125001298. J Endotoxin Res. 2003. PMID: 12691614 Review.
-
Interleukin-1-induced plasticity of hypothalamic CRH neurons and long-term stress hyperresponsiveness.Ann N Y Acad Sci. 1998 May 1;840:65-73. doi: 10.1111/j.1749-6632.1998.tb09550.x. Ann N Y Acad Sci. 1998. PMID: 9629238 Review.
Cited by
-
Hypothalamic integration of immune function and metabolism.Prog Brain Res. 2006;153:367-405. doi: 10.1016/S0079-6123(06)53022-5. Prog Brain Res. 2006. PMID: 16876587 Free PMC article. Review.
-
Leptin fails to blunt the lipopolysaccharide-induced activation of the hypothalamic-pituitary-adrenal axis in rats.J Endocrinol. 2014 Apr 22;221(2):229-34. doi: 10.1530/JOE-13-0249. Print 2014 May. J Endocrinol. 2014. PMID: 24578293 Free PMC article.
-
Pubertal changes in the pituitary and adrenal glands of male and female rats: Relevance to stress reactivity.Neurobiol Stress. 2022 May 6;18:100457. doi: 10.1016/j.ynstr.2022.100457. eCollection 2022 May. Neurobiol Stress. 2022. PMID: 35592027 Free PMC article.
-
Lipopolysaccharide increases plasma levels of corticotropin-releasing hormone in rats.Neuroendocrinology. 2011;93(3):165-73. doi: 10.1159/000322590. Epub 2010 Dec 6. Neuroendocrinology. 2011. PMID: 21135542 Free PMC article.
-
Cherry Juice Improves Memory and Anxiety by Modulating Cell Number in the Hippocampus of Male Rat Pups Infected with Lipopolysaccharides During Gestation and Gestation-Lactation.Int J Mol Sci. 2025 Jun 12;26(12):5642. doi: 10.3390/ijms26125642. Int J Mol Sci. 2025. PMID: 40565105 Free PMC article.
References
-
- Ban E, Milon G, Prudhomme N, Fillion G, Haour F. Receptors for interleukin-1 (α and β) in mouse brain: mapping and neuronal localization in hippocampus. Neuroscience. 1991;43:21–30. - PubMed
-
- Bohler HC, Zoeller RT, King JC, Rubin BS, Weber R, Merriam GR. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Mol Brain Res. 1990;8:259–262. - PubMed
-
- Broad KD, Keverne EB, Kendrick KM. Corticotropin releasing factor mRNA expression in the sheep brain during pregnancy, parturition and lactation and following exogenous progesterone and oestrogen treatment. Mol Brain Res. 1995;29:310–316. - PubMed
-
- Broadwell RD, Brightman MW. Entry of peroxidase into neurons of the central and peripheral nervous systems from extracerebral and cerebral blood. J Comp Neurol. 1976;166:257–283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

