Endothelial nitric oxide synthase, caveolae and the development of atherosclerosis
- PMID: 12562964
- PMCID: PMC2342632
- DOI: 10.1113/jphysiol.2002.031534
Endothelial nitric oxide synthase, caveolae and the development of atherosclerosis
Abstract
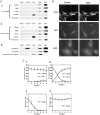
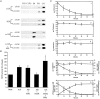
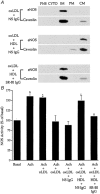
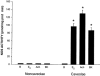
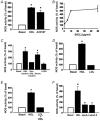
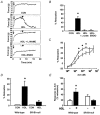
Early hypercholesterolaemia-induced vascular disease is characterized by an attenuated capacity for endothelial production of the antiatherogenic molecule nitric oxide (NO), which is generated by endothelial NO synthase (eNOS). In recent studies we have determined the impact of lipoproteins on eNOS subcellular localization and action, thereby providing a causal link between cholesterol status and initial abnormalities in endothelial function. We have demonstrated that eNOS is normally targeted to cholesterol-enriched caveolae where it resides in a signalling module. Oxidized low density lipoprotein (LDL; oxLDL) causes displacement of eNOS from caveolae by binding to endothelial cell CD36 receptors and by depleting caveolae cholesterol content, resulting in the disruption of eNOS activation. The adverse effects of oxLDL are fully prevented by high density lipoprotein (HDL) via binding to scavenger receptor BI (SR-BI), which is colocalized with eNOS in endothelial caveolae. This occurs through the maintenance of caveolae cholesterol content by cholesterol ester uptake from HDL. As importantly, HDL binding to SR-BI causes robust stimulation of eNOS activity in endothelial cells, and this process is further demonstrable in isolated endothelial cell caveolae. HDL also enhances endothelium- and NO-dependent relaxation in aortae from wild-type mice, but not in aortae from homozygous null SR-BI knockout mice. Thus, lipoproteins have potent effects on eNOS function in caveolae via actions on both membrane cholesterol homeostasis and the level of activation of the enzyme. These processes may be critically involved in the earliest phases of atherogenesis, which recent studies suggest may occur during fetal life.
Figures

References
-
- Anderson RG, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. - PubMed
-
- Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem. 1999;274:32512–32519. - PubMed
-
- Cayatte AJ, Palacino JJ, Horten K, Cohen RA. Chronic inhibition of nitric oxide production accelerates neointima formation and impairs endothelial function in hypercholesterolemic rabbits. Arterioscler Thromb. 1994;14:753–759. - PubMed
-
- Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul P. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

