Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro
- PMID: 12562965
- PMCID: PMC2342622
- DOI: 10.1113/jphysiol.2002.031450
Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro
Abstract
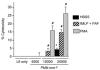
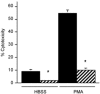
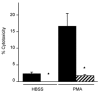
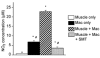
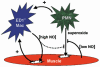
Current evidence indicates that the physiological functions of inflammatory cells are highly sensitive to their microenvironment, which is partially determined by the inflammatory cells and their potential targets. In the present investigation, interactions between neutrophils, macrophages and muscle cells that may influence muscle cell death are examined. Findings show that in the absence of macrophages, neutrophils kill muscle cells in vitro by superoxide-dependent mechanisms, and that low concentrations of nitric oxide (NO) protect against neutrophil-mediated killing. In the absence of neutrophils, macrophages kill muscle cells through a NO-dependent mechanism, and the presence of target muscle cells causes a three-fold increase in NO production by macrophages, with no change in the concentration of inducible nitric oxide synthase. Muscle cells that are co-cultured with both neutrophils and macrophages in proportions that are observed in injured muscle show cytotoxicity through a NO-dependent, superoxide-independent mechanism. Furthermore, the concentration of myeloid cells that is necessary for muscle killing is greatly reduced in assays that use mixed myeloid cell populations, rather than uniform populations of neutrophils or macrophages. These findings collectively show that the magnitude and mechanism of muscle cell killing by myeloid cells are modified by interactions between muscle cells and neutrophils, between muscle cells and macrophages and between macrophages and neutrophils.
Figures




References
-
- Abu-Soud HM, Hazen SL. Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem. 2000;275:5425–5430. - PubMed
-
- Amano F, Noda T. Improved detection of nitric oxide radical (NO) production in an activated macrophage culture with a radical scavenger, carboxy PTIO, and Griess reagent. FEBS Letters. 1995;368:425–428. - PubMed
-
- Blaylock MG, Cuthbertson BH, Galley HF, Ferguson N, Webster NR. The effect of nitric oxide and peroxynitrite on apoptosis in human polymorphonuclear leukocytes. Free Rad Biol Med. 1998;25:748–752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

