Somatic exocytosis of serotonin mediated by L-type calcium channels in cultured leech neurones
- PMID: 12562971
- PMCID: PMC2342656
- DOI: 10.1113/jphysiol.2002.030684
Somatic exocytosis of serotonin mediated by L-type calcium channels in cultured leech neurones
Abstract
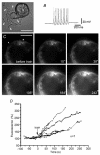
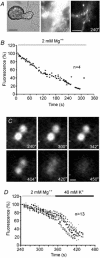
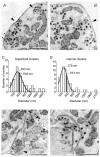
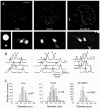
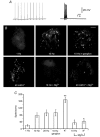
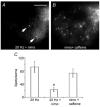
We studied somatic exocytosis of serotonin and its mediation by L-type calcium (Ca2+) channels in cultured Retzius neurones of the leech. Exocytosis was induced by trains of impulses at different frequencies or by depolarisation with 40 mM potassium (K+), and was quantified by use of the fluorescent dye FM 1-43. Stimulation increased the membrane fluorescence and produced a pattern of FM 1-43 fluorescent spots of 1.28 +/- 0.01 microm in diameter, provided that Ca2+ was present in the bathing fluid. Individual spots lost their stain during depolarisation with 40 mM K+. Electron micrographs showed clusters of dense core vesicles, some of which were in contact with the cell membrane. Presynaptic structures with clear vesicles were absent from the soma. The number of fluorescent spots per soma, but not their diameter or their fluorescence intensity, depended on the frequency of stimulation. Trains at 1 Hz produced 19.5 +/- 5 spots per soma, 77.9 +/- 13.9 spots per soma were produced at 10 Hz and 91.5 +/- 16.9 spots per soma at 20 Hz. Staining patterns were similar for neurones in culture and in situ. In the presence of the L-type Ca2+ channel blocker nimodipine (10 microM), a 20 Hz train produced only 22.9 +/- 6.4 spots per soma, representing a 75 % reduction compared to control cells (P < 0.05). Subsequent incubation with 10 mM caffeine to induce Ca2+ release from intracellular stores increased the number of spots to 73.22 +/- 12.5. Blockers of N-, P-, Q- or invertebrate Ca2+ channels did not affect somatic exocytosis. Our results suggest that somatic exocytosis by neurones shares common mechanisms with excitable endocrine cells.
Figures
References
-
- Angleson JK, Cochilla AJ, Kilic G, Nussinovitch I, Betz WJ. Regulation of dense core release from neuroendocrine cells revealed by imaging single exocytic events. Nat Neurosci. 1999;2:440–446. - PubMed
-
- Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. - PubMed
-
- Beck A, Lohr C, Deitmer JW. Calcium transients in subcompartments of the leech Retzius neuron as induced by single action potentials. J Neurobiol. 2001;48:1–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

