Temperature and redox state dependence of native Kv2.1 currents in rat pancreatic beta-cells
- PMID: 12562993
- PMCID: PMC2342601
- DOI: 10.1113/jphysiol.2002.035709
Temperature and redox state dependence of native Kv2.1 currents in rat pancreatic beta-cells
Abstract
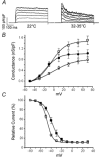
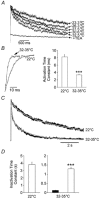
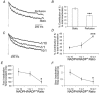
In pancreatic beta-cells, voltage-dependent K(+) (Kv) channels repolarise glucose-stimulated action potentials. Kv channels are therefore negative regulators of Ca(2+) entry and insulin secretion. We have recently demonstrated that Kv2.1 mediates the majority of beta-cell voltage-dependent outward K(+) current and now investigate the function of native beta-cell Kv2.1 channels at near-physiological temperatures (32-35 degrees C). While beta-cell voltage-dependent outward K(+) currents inactivated little at room temperature, both fast-inactivation (111.5 +/- 14.3 ms) and slow-inactivation (1.21 +/- 0.12 s) was observed at 32-35 degrees C. Kv2.1 mediates the fast-inactivating current observed at 32-35 degrees C, since it could be selectively ablated by expression of a dominant-negative Kv2.1 construct (Kv2.1N). The surprising ability of Kv2.1N to selectively remove the fast-inactivating component, together with its sensitivity to tetraethylammonium (TEA), demonstrate that this component is not mediated by the classically fast-inactivating and TEA-resistant channels such as Kv1.4 and 4.2. Increasing the intracellular redox state by elevating the cytosolic NADPH/NADP(+) ratio from 1/10 to 10/1 increased the rates of both fast- and slow-inactivation. In addition, increasing the intracellular redox state also increased the relative contribution of the fast-inactivation component from 38.8 +/- 2.1 % to 55.9 +/- 1.8 %. The present study suggests that, in beta-cells, Kv2.1 channels mediate a fast-inactivating K(+) current at physiological temperatures and may be regulated by the metabolic generation of NADPH.
Figures

Similar articles
-
Members of the Kv1 and Kv2 voltage-dependent K(+) channel families regulate insulin secretion.Mol Endocrinol. 2001 Aug;15(8):1423-35. doi: 10.1210/mend.15.8.0685. Mol Endocrinol. 2001. PMID: 11463864
-
Inhibition of Kv2.1 voltage-dependent K+ channels in pancreatic beta-cells enhances glucose-dependent insulin secretion.J Biol Chem. 2002 Nov 22;277(47):44938-45. doi: 10.1074/jbc.M205532200. Epub 2002 Sep 20. J Biol Chem. 2002. PMID: 12270920
-
Molecular diversity of the repolarizing voltage-gated K+ currents in mouse atrial cells.J Physiol. 2000 Dec 1;529 Pt 2(Pt 2):345-58. doi: 10.1111/j.1469-7793.2000.00345.x. J Physiol. 2000. PMID: 11101645 Free PMC article.
-
Beta-cell ion channels: keys to endodermal excitability.Horm Metab Res. 1999 Aug;31(8):455-61. doi: 10.1055/s-2007-978774. Horm Metab Res. 1999. PMID: 10494870 Review.
-
Voltage-dependent K(+) channels in pancreatic beta cells: role, regulation and potential as therapeutic targets.Diabetologia. 2003 Aug;46(8):1046-62. doi: 10.1007/s00125-003-1159-8. Epub 2003 Jun 27. Diabetologia. 2003. PMID: 12830383 Review.
Cited by
-
Regulation of ion channels by pyridine nucleotides.Circ Res. 2013 Feb 15;112(4):721-41. doi: 10.1161/CIRCRESAHA.111.247940. Circ Res. 2013. PMID: 23410881 Free PMC article. Review.
-
Glucose-sensing mechanisms in pancreatic beta-cells.Philos Trans R Soc Lond B Biol Sci. 2005 Dec 29;360(1464):2211-25. doi: 10.1098/rstb.2005.1762. Philos Trans R Soc Lond B Biol Sci. 2005. PMID: 16321791 Free PMC article. Review.
-
Ionic mechanisms and Ca2+ dynamics underlying the glucose response of pancreatic β cells: a simulation study.J Gen Physiol. 2011 Jul;138(1):21-37. doi: 10.1085/jgp.201110611. J Gen Physiol. 2011. PMID: 21708953 Free PMC article.
-
Metabolic cycling in control of glucose-stimulated insulin secretion.Am J Physiol Endocrinol Metab. 2008 Dec;295(6):E1287-97. doi: 10.1152/ajpendo.90604.2008. Epub 2008 Aug 26. Am J Physiol Endocrinol Metab. 2008. PMID: 18728221 Free PMC article. Review.
-
Ionic mechanisms in pancreatic β cell signaling.Cell Mol Life Sci. 2014 Nov;71(21):4149-77. doi: 10.1007/s00018-014-1680-6. Epub 2014 Jul 23. Cell Mol Life Sci. 2014. PMID: 25052376 Free PMC article. Review.
References
-
- Ammon HP, Bacher M, Brandle WF, Waheed A, Roenfeldt M, el Sayed ME, Ahmed AA, Wahl MA. Effect of forty-eight-hour glucose infusion into rats on islet ion fluxes, ATP/ADP ratio and redox ratios of pyridine nucleotides. J Endocrinol. 1998;156:583–590. - PubMed
-
- Ammon HP, Wahl MA. Islet redox ratios: Their role in insulin release. In: Flatt PR, Lenzen S, editors. Frontiers of Insulin Secretion and Pancreatic β-cell Research. London: Smith-Gordon; 1994. pp. 112–113.
-
- Archer SL, Weir EK, Reeve HL, Michelakis E. Molecular identification of O2 sensors and O2-sensitive potassium channels in the pulmonary circulation. Adv Exp Med Biol. 2000;475:219–240. - PubMed
-
- Bahring R, Milligan CJ, Vardanyan V, Engeland B, Young BA, Dannenberg J, Waldschutz R, Edwards JP, Wray D, Pongs O. Coupling of voltage-dependent potassium channel inactivation and oxidoreductase active site of Kvβ subunits. J Biol Chem. 2001;276:22923–22929. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

