Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP
- PMID: 12563000
- PMCID: PMC2342584
- DOI: 10.1113/jphysiol.2002.028704
Oscillations in ciliary beat frequency and intracellular calcium concentration in rabbit tracheal epithelial cells induced by ATP
Abstract
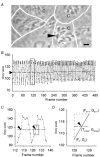
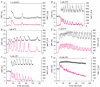
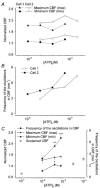
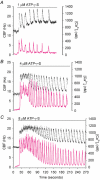
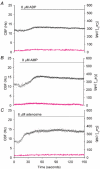
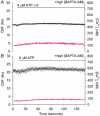
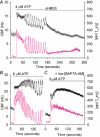
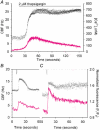
To investigate how Ca(2+) regulates airway ciliary activity, changes in ciliary beat frequency (CBF) and intracellular calcium concentration ([Ca(2+)](i)) of rabbit tracheal ciliated cells, in response to ATP, were simultaneously quantified with high-speed phase-contrast and fast fluorescence imaging. [ATP]<or= 1 microM induced an increase in [Ca(2+)](i) and CBF that declined to the initial basal levels and was followed by irregular brief increases in [Ca(2+)](i) and CBF. [ATP] > 1 but < 16 microM induced a similar increase in [Ca(2+)](i) and CBF but this was followed by oscillations in CBF and [Ca(2+)](i). The minimum CBF of the oscillations in CBF remained elevated above the basal rate while the minimum concentration of the [Ca(2+)](i) oscillations returned to the basal level. The minimum and maximum CBF of the oscillations in CBF were independent of the [ATP], whereas the frequency of the oscillations in CBF was dependent on the [ATP]. Similar oscillations in CBF and [Ca(2+)](i) were induced by ATP- gamma -S. Although ADP, AMP and adenosine induced a Ca(2+)-independent increase in CBF, neither ATP nor ATP- gamma -S induced an increase in CBF when the Ca(2+) increases were abolished by 20 microM BAPTA AM, a result suggesting that ATP hydrolysis was minimal. [ATP] >or=16 microM induced a sustained elevation in CBF and only a temporary, non-oscillating increase in [Ca(2+)](i). A similar response was induced by thapsigargin (2 microM). Flash photolysis of caged Ca(2+) (NP-EGTA) produced both transient and prolonged increases in [Ca(2+)](i) which were accompanied by transient and sustained increases in CBF, respectively. From these results, we propose that CBF can be increased by a direct Ca(2+) -dependent mechanism that generates the rapid increases in CBF associated with the oscillations or by an indirect Ca(2+)-dependent mechanism that is responsible for the sustained minimum increase in CBF.
Figures


References
-
- Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
-
- Berridge MJ. The AM and FM of calcium signalling. Nature. 1997;386:759–760. - PubMed
-
- Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. - PubMed
-
- Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, Mackenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling-an overview. Semin Cell Dev Biol. 2001b;12:3–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

