Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum
- PMID: 12563001
- PMCID: PMC2342587
- DOI: 10.1113/jphysiol.2002.033365
Involvement of intramuscular interstitial cells in nitrergic inhibition in the mouse gastric antrum
Abstract
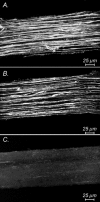
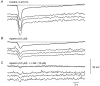
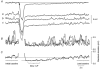
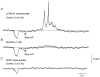
Intracellular recordings were made from isolated bundles of the circular muscle layer of mouse gastric antrum and the responses evoked by stimulating intrinsic nerve fibres were examined. Transmural nerve stimulation evoked a fast inhibitory junction potential (fast-IJP) which was followed initially by a smaller amplitude long lasting inhibitory junction potential (slow-IJP) and a period of excitation. The excitatory component of the response was abolished by atropine, suggesting that it resulted from the release of acetylcholine and activation of muscarinic receptors. Fast-IJPs were selectively reduced in amplitude by apamin and slow-IJPs were abolished by N(omega)-nitro-L-arginine. Slow-IJPs were associated with a drop in membrane noise, suggesting that inhibition resulted from a reduced discharge of unitary potentials by intramuscular interstitial cells of Cajal (ICC(IM)). The chloride channel blocker, anthracene-9-carboxylic acid, reduced the discharge of membrane noise in a manner similar to that detected during the slow-IJP. When recordings were made from the antrum of W/W(V) mice, which lack ICC(IM), the cholinergic and nitrergic components were absent, with only fast-IJPs being detected. The observations suggest that neurally released nitric oxide selectively targets ICC(IM) causing a hyperpolarization by suppressing the discharge of unitary potentials.
Figures





References
-
- Benham CD, Bolton TB, Lang RJ. Acetylcholine activates an inward current in single mammalian smooth muscle cells. Nature. 1985;328:275–278. - PubMed
-
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-kit immunohistochemistry. Cell Tissue Res. 1997;290:11–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

