Essential role of rho kinase in the Ca2+ sensitization of prostaglandin F(2alpha)-induced contraction of rabbit aortae
- PMID: 12563007
- PMCID: PMC2342586
- DOI: 10.1113/jphysiol.2002.030775
Essential role of rho kinase in the Ca2+ sensitization of prostaglandin F(2alpha)-induced contraction of rabbit aortae
Abstract
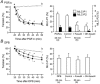
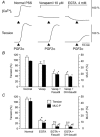
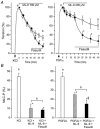
Inhibition of dephosphorylation of the 20 kDa myosin light chain (MLC(20)) is an important mechanism for the Ca(2+)-induced sensitization of vascular smooth muscle contraction. We investigated whether this mechanism operates in prostaglandin F(2alpha) (PGF(2alpha))-induced contraction of rabbit aortic smooth muscle and, if so, whether protein kinase C (PKC) or rho-associated kinase (rho kinase) contribute to the inhibition of dephosphorylation. In normal medium, PGF(2alpha) (10 microM) increased the phosphorylation of MLC(20) and developed tension. The rho-kinase inhibitors fasudil and hydroxyfasudil inhibited these changes, despite having no effect on a phorbol-ester-induced MLC(20) phosphorylation. After treatment with verapamil or chelation of external Ca(2+) with EGTA, PGF(2alpha) increased the MLC(20) phosphorylation and tension without an increase in [Ca(2+)](i), all of which were sensitive to fasudil and hydroxyfasudil. ML-9, a MLC kinase inhibitor, quickly reversed the KCl-induced MLC(20) phosphorylation and contraction to the resting level. However, fractions of PGF(2alpha)-induced contraction and MLC(20) phosphorylation were resistant to ML-9 but were sensitive to fasudil. Ro31-8220 (10 microM), a PKC inhibitor, did not affect the phosphorylation of MLC(20) and the tension caused by PGF(2alpha), thus excluding the possibility of the involvement of PKC in the PGF(2alpha)-induced MLC(20) phosphorylation. PGF(2alpha) increased phosphorylation at Thr654 of the myosin binding subunit (MBS) of myosin phosphatase, which is a target of rho kinase, and fasudil decreased the phosphorylation. These data suggest that the PGF(2alpha)-induced contraction is accompanied by the inhibition of MLC(20) dephosphorylation through rho kinase-induced MBS phosphorylation, leading to Ca(2+) sensitization of contraction. An actin-associated mechanism may also be involved in the PGF(2alpha)-induced sensitization.
Figures





Similar articles
-
Inhibition of protein kinase C-mediated contraction by Rho kinase inhibitor fasudil in rabbit aorta.Naunyn Schmiedebergs Arch Pharmacol. 2004 Nov;370(5):414-22. doi: 10.1007/s00210-004-0975-9. Epub 2004 Oct 1. Naunyn Schmiedebergs Arch Pharmacol. 2004. PMID: 15459803
-
A role for Rho-kinase in Ca-independent contractions induced by phorbol-12,13-dibutyrate.Clin Exp Pharmacol Physiol. 2009 Mar;36(3):256-61. doi: 10.1111/j.1440-1681.2008.05045.x. Epub 2008 Oct 8. Clin Exp Pharmacol Physiol. 2009. PMID: 18986333
-
Ca2+-dependent rapid Ca2+ sensitization of contraction in arterial smooth muscle.Circ Res. 2007 Jan 5;100(1):121-9. doi: 10.1161/01.RES.0000253902.90489.df. Epub 2006 Dec 7. Circ Res. 2007. PMID: 17158339 Free PMC article.
-
[The molecular mechanism of vasospasm and the attenuation by fasudil].Nihon Yakurigaku Zasshi. 1999 Oct;114 Suppl 1:66P-70P. doi: 10.1254/fpj.114.supplement_66. Nihon Yakurigaku Zasshi. 1999. PMID: 10629857 Review. Japanese.
-
Rho-Mancing to Sensitize Calcium Signaling for Contraction in the Vasculature: Role of Rho Kinase.Adv Pharmacol. 2017;78:303-322. doi: 10.1016/bs.apha.2016.09.001. Epub 2016 Oct 27. Adv Pharmacol. 2017. PMID: 28212799 Review.
Cited by
-
MYPT1 isoforms expressed in HEK293T cells are differentially phosphorylated after GTPγS treatment.J Smooth Muscle Res. 2016;52(0):66-77. doi: 10.1540/jsmr.52.66. J Smooth Muscle Res. 2016. PMID: 27725371 Free PMC article.
-
Enhancement of myofilament calcium sensitivity by acute hypoxia in rat distal pulmonary arteries.Am J Physiol Lung Cell Mol Physiol. 2011 Sep;301(3):L380-7. doi: 10.1152/ajplung.00068.2011. Epub 2011 Jun 10. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21665962 Free PMC article.
-
Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo.Am J Physiol Cell Physiol. 2008 Aug;295(2):C358-64. doi: 10.1152/ajpcell.90645.2007. Epub 2008 Jun 4. Am J Physiol Cell Physiol. 2008. PMID: 18524939 Free PMC article.
-
Rho-kinase-mediated regulation of receptor-agonist-stimulated smooth muscle contraction.Pflugers Arch. 2006 Nov;453(2):223-32. doi: 10.1007/s00424-006-0133-y. Epub 2006 Sep 5. Pflugers Arch. 2006. PMID: 16953424
-
Rho kinase (ROCK) inhibitors.J Cardiovasc Pharmacol. 2007 Jul;50(1):17-24. doi: 10.1097/FJC.0b013e318070d1bd. J Cardiovasc Pharmacol. 2007. PMID: 17666911 Free PMC article. Review.
References
-
- Beavo JA, Bechtel PJ, Krebs EG. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. - PubMed
-
- Chehrazi BB, Giri S, Joy RM. Prostaglandins and vasoactive amines in cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 1989;20:217–224. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

