PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex
- PMID: 12563010
- PMCID: PMC2342599
- DOI: 10.1113/jphysiol.2002.031369
PSD-95 regulates synaptic transmission and plasticity in rat cerebral cortex
Abstract
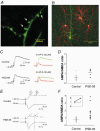
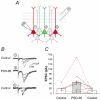
PSD-95 is one of the most abundant proteins found in the postsynaptic density of excitatory synapses. However, the precise functional role played by PSD-95 in regulating synaptic transmission and plasticity remains undefined. To address this issue, we have overexpressed PSD-95 in cortical pyramidal neurons in organotypic brain slices using particle-mediated gene transfer and assessed the consequences on synaptic transmission and plasticity. The AMPA receptor/NMDA receptor (AMPAR/NMDAR) ratio of evoked EPSCs recorded at +40 mV was greater in PSD-95-transfected pyramidal neurons than in controls. This difference could not be accounted for by a change in rectification of AMPAR-mediated synaptic currents since the current-voltage curves obtained in controls and in PSD-95-transfected neurons were indistinguishable. However, the amplitude of AMPAR-mediated evoked EPSCs was larger in PSD-95-transfected neurons compared to matched controls. Paired-pulse ratio analysis suggested that overexpression of PSD-95 did not alter presynaptic release probability. Transfection of PSD-95 was further accompanied by an increase in the frequency, but not amplitude, of AMPAR-mediated mEPSCs. Together, these results indicate that transfection of PSD-95 increased AMPAR-mediated synaptic transmission. Furthermore, they suggest that this phenomenon reflects an increased number of synapses expressing AMPARs rather than an increased number or function of these receptors at individual synapses. We tested the consequences of these changes on synaptic plasticity and found that PSD-95 transfection greatly enhanced the probability of observing long-term depression. These results thus identify a physiological role for PSD-95 and demonstrate that this protein can play a decisive role in controlling synaptic strength and activity-dependent synaptic plasticity.
Figures

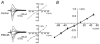
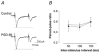
References
-
- Craven SE, Bredt DS. PDZ proteins organize synaptic signaling pathways. Cell. 1998;93:495–498. - PubMed
-
- Edmonds B, Gibb AJ, Colquhoun D. Mechanisms of activation of glutamate receptors and the time course of excitatory synaptic currents. Annu Rev Physiol. 1995;57:495–519. - PubMed
-
- El Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. - PubMed
-
- El Husseini AE, Schnell E, Dakoji S, Sweeney N, Zhou Q, Prange O, Gauthier-Campbell C, Aguilera-Moreno A, Nicoll RA, Bredt DS. Synaptic strength regulated by palmitate cycling on PSD-95. Cell. 2002;108:849–863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

