Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells
- PMID: 12563034
- PMCID: PMC149872
- DOI: 10.1073/pnas.0337677100
Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells
Abstract
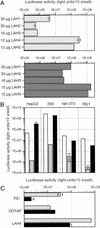
Gene delivery has shown potential in a wide variety of applications, including basic research, therapies for genetic and acquired diseases, and vaccination. Most available nonviral systems have serious drawbacks such as the inability to control and scale the production process in a reproducible manner. Here, we demonstrate a biotechnologically feasible approach for gene delivery, using synthetic cationic amphipathic peptides containing a variable number of histidine residues. Gene transfer to different cell lines in vitro was achieved with an efficiency comparable to commercially available reagents. We provide evidence that the transfection efficiency depends on the number and positioning of histidine residues in the peptide as well as on the pH at which the in-plane to transmembrane transition takes place. Endosomal acidification is also required. Interestingly, even when complexed to DNA these peptides maintain a high level of antibacterial activity, opening the possibility of treating the genetic defect and the bacterial infections associated with cystic fibrosis with a single compound. Thus, this family of peptides represents a new class of agents that may have broad utility for gene transfer and gene therapy applications.
Figures


Similar articles
-
An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection.Biomaterials. 2008 May;29(15):2408-14. doi: 10.1016/j.biomaterials.2008.01.031. Epub 2008 Mar 4. Biomaterials. 2008. PMID: 18295328
-
Efficient gene transfer by histidylated polylysine/pDNA complexes.Bioconjug Chem. 1999 May-Jun;10(3):406-11. doi: 10.1021/bc9801070. Bioconjug Chem. 1999. PMID: 10346871
-
Cationic amphipathic histidine-rich peptides for gene delivery.Biochim Biophys Acta. 2006 Mar;1758(3):301-7. doi: 10.1016/j.bbamem.2006.02.005. Epub 2006 Feb 28. Biochim Biophys Acta. 2006. PMID: 16540079 Review.
-
Preparation and in vitro evaluation of novel lipopeptide transfection agents for efficient gene delivery.Bioconjug Chem. 2008 Apr;19(4):940-50. doi: 10.1021/bc700463q. Epub 2008 Mar 12. Bioconjug Chem. 2008. PMID: 18333604
-
GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery.Adv Drug Deliv Rev. 2004 Apr 23;56(7):967-85. doi: 10.1016/j.addr.2003.10.041. Adv Drug Deliv Rev. 2004. PMID: 15066755 Review.
Cited by
-
Peptides in cancer nanomedicine: drug carriers, targeting ligands and protease substrates.J Control Release. 2012 Apr 10;159(1):2-13. doi: 10.1016/j.jconrel.2011.10.023. Epub 2011 Oct 26. J Control Release. 2012. PMID: 22056916 Free PMC article. Review.
-
Tumor-targeted RNA-interference: functional non-viral nanovectors.Am J Cancer Res. 2011 Sep 1;1(1):25-42. Am J Cancer Res. 2011. PMID: 21572539 Free PMC article.
-
A Gemini Cationic Lipid with Histidine Residues as a Novel Lipid-Based Gene Nanocarrier: A Biophysical and Biochemical Study.Nanomaterials (Basel). 2018 Dec 16;8(12):1061. doi: 10.3390/nano8121061. Nanomaterials (Basel). 2018. PMID: 30558369 Free PMC article.
-
Amphipathic Cell-Penetrating Peptide-Aided Delivery of Cas9 RNP for In Vitro Gene Editing and Correction.Pharmaceutics. 2023 Oct 20;15(10):2500. doi: 10.3390/pharmaceutics15102500. Pharmaceutics. 2023. PMID: 37896260 Free PMC article.
-
Curcumin-loaded nanoliposomes linked to homing peptides for integrin targeting and neuropilin-1-mediated internalization.Pharm Biol. 2017 Dec;55(1):277-285. doi: 10.1080/13880209.2016.1261301. Pharm Biol. 2017. PMID: 27937055 Free PMC article.
References
-
- Mahato R I, Monera O D, Smith L C, Rolland A. Curr Opin Mol Ther. 1999;1:226–243. - PubMed
-
- Harbottle R P, Cooper R G, Hart S L, Ladhoff A, McKay T, Knight A M, Wagner E, Miller A D, Coutelle C. Hum Gene Ther. 1998;9:1037–1047. - PubMed
-
- Plank C, Oberhauser B, Mechtler K, Koch C, Wagner E. J Biol Chem. 1994;269:12918–12924. - PubMed
-
- Wyman T B, Nicol F, Zelphati O, Scaria P V, Plank C, Szoka F C., Jr Biochemistry. 1997;36:3008–3017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

