The strategy for coupling the RanGTP gradient to nuclear protein export
- PMID: 12563037
- PMCID: PMC149899
- DOI: 10.1073/pnas.252766999
The strategy for coupling the RanGTP gradient to nuclear protein export
Abstract
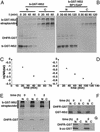
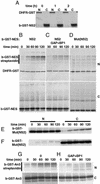
The Ran GTPase plays critical roles in both providing energy for and determining the directionality of nucleocytoplasmic transport. The mechanism that couples the RanGTP gradient to nuclear protein export will determine the rate of and limits to accumulation of export cargoes in the cytoplasm, but is presently unknown. We reasoned that plausible coupling mechanisms could be distinguished by comparing the rates of reverse motion of export cargoes through the nuclear pore complex (NPC) with the predictions of a mathematical model. Measurement of reverse export rates in Xenopus oocytes revealed that nuclear export signals can facilitate RanGTP-dependent cargo movement into the nucleus against the RanGTP gradient at rates comparable to export rates. Although export cargoes with high affinity for their receptor are exported faster than those with low affinity, their reverse transport is also greater. The ratio of the rates of reverse and forward export of a cargo is proportional to its rate of diffusion through the NPC, i.e., to the ability of the cargo to penetrate the NPC permeability barrier. The data substantiate a diffusional mechanism of coupling and suggest the existence of a high concentration of RanGTP-receptor complexes within the NPC that decreases sharply at the cytoplasmic boundary of the NPC permeability barrier.
Figures



References
-
- Mattaj I W, Englmeier L. Annu Rev Biochem. 1998;67:265–306. - PubMed
-
- Gorlich D, Kutay U. Annu Rev Cell Dev Biol. 1999;15:607–660. - PubMed
-
- Fornerod M, Ohno M, Yoshida M, Mattaj I W. Cell. 1997;90:1051–1060. - PubMed
-
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. Nature. 1997;390:308–311. - PubMed
-
- Stade K, Ford C S, Guthrie C, Weis K. Cell. 1997;90:1041–1050. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

