Inhibition of subliminally primed responses is mediated by the caudate and thalamus: evidence from functional MRI and Huntington's disease
- PMID: 12566291
- PMCID: PMC3838934
- DOI: 10.1093/brain/awg067
Inhibition of subliminally primed responses is mediated by the caudate and thalamus: evidence from functional MRI and Huntington's disease
Abstract
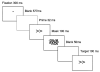
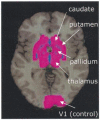
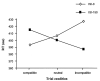
Masked prime tasks have shown that sensory information that has not been consciously perceived can nevertheless trigger the preactivation of a motor response. Automatic inhibitory control processes prevent such response tendencies from interfering with behaviour. The present study investigated the possibility that these inhibitory control processes are mediated by a cortico-striatal-pallidal-thalamic pathway by using a masked prime task with Huntington's disease patients (Experiment 1) and with healthy volunteers in a functional MRI (fMRI) study (Experiment 2). In the masked prime task, clearly visible left- or right-pointing target arrows are preceded by briefly presented and subsequently masked prime arrows. Participants respond quickly with a left or right key-press to each target. Trials are either compatible (prime and target pointing in the same direction) or incompatible (prime and target pointing in different directions). Prior behavioural and electrophysiological results show that automatic inhibition of the initially primed response tendency is reflected in a 'negative compatibility effect' (faster reaction times for incompatible trials than for compatible trials), and is shown to consist of three distinct processes (prime activation, response inhibition and response conflict) occurring within 300 ms. Experiment 1 tested the hypothesis that lesions of the striatum would interrupt automatic inhibitory control by studying early-stage Huntington's disease patients. Findings supported the hypothesis: there was a bimodal distribution for patients, with one-third (choreic) showing disinhibition, manifested as an absent negative compatibility effect, and two-thirds (non-choreic) showing excessive inhibition, manifested as a significantly greater negative compatibility effect than that in controls. Experiment 2 used fMRI and a region of interest (ROI) template-based method to further test the hypothesis that structures of the striatal-pallidal-thalamic pathway mediate one or more of the processes of automatic inhibitory control. Neither prime activation nor response conflict significantly engaged any ROIs, but the response inhibition process led to significant modulation of both the caudate and thalamus. Taken together, these experiments indicate a causal role for the caudate nucleus and thalamus in automatic inhibitory motor control, and the results are consistent with performance of the task requiring both direct and indirect striatal-pallidal-thalamic pathways. The finding that Huntington's disease patients with greater chorea were disinhibited is consistent with the theory that chorea arises from selective degeneration of striatal projections to the lateral globus pallidus, while the exaggerated inhibitory effect for patients with little or no chorea may be due to additional degeneration of projections to the medial globus pallidus.
Figures

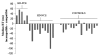
References
-
- Albin RL, Reiner A, Anderson KD, Penney JB, Young AB. Striatal and nigral neuron subpopulations in rigid Huntington’s disease: implications for the functional anatomy of chorea and rigidity-akinesia. Ann Neurol. 1990;27:357–65. - PubMed
-
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, ‘prefrontal’ and ‘limbic’ functions. [Review] Prog Brain Res. 1990;85:119–46. - PubMed
-
- Beiser DG, Hua SE, Houk JC. Network models of the basal ganglia. [Review] Curr Opin Neurobiol. 1997;7:185–90. - PubMed
-
- Bruyn GW, Went LN. Huntington’s chorea. In: Vinken PJ, Bruyn GW, Klawans HL, editors. Handbook of clinical neurology. Vol. 49. Elsevier; Amsterdam: 1986. pp. 267–313.
-
- Coles MG, Gratton G, Bashore TR, Eriksen CW, Donchin E. A psychophysiological investigation of the continuous flow model of human information processing. J Exp Psychol Hum Percept Perform. 1985;11:529–53. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

