Global RNA half-life analysis in Escherichia coli reveals positional patterns of transcript degradation
- PMID: 12566399
- PMCID: PMC420366
- DOI: 10.1101/gr.912603
Global RNA half-life analysis in Escherichia coli reveals positional patterns of transcript degradation
Abstract
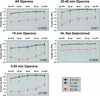
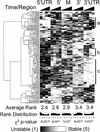
Subgenic-resolution oligonucleotide microarrays were used to study global RNA degradation in wild-type Escherichia coli MG1655. RNA chemical half-lives were measured for 1036 open reading frames (ORFs) and for 329 known and predicted operons. The half-life of total mRNA was 6.8 min under the conditions tested. We also observed significant relationships between gene functional assignments and transcript stability. Unexpectedly, transcription of a single operon (tdcABCDEFG) was relatively rifampicin-insensitive and showed significant increases 2.5 min after rifampicin addition. This supports a novel mechanism of transcription for the tdc operon, whose promoter lacks any recognizable sigma binding sites. Probe by probe analysis of all known and predicted operons showed that the 5' ends of operons degrade, on average, more quickly than the rest of the transcript, with stability increasing in a 3' direction, supporting and further generalizing the current model of a net 5' to 3' directionality of degradation. Hierarchical clustering analysis of operon degradation patterns revealed that this pattern predominates but is not exclusive. We found a weak but highly significant correlation between the degradation of adjacent operon regions, suggesting that stability is determined by a combination of local and operon-wide stability determinants. The 16 ORF dcw gene cluster, which has a complex promoter structure and a partially characterized degradation pattern, was studied at high resolution, allowing a detailed and integrated description of its abundance and degradation. We discuss the application of subgenic resolution DNA microarray analysis to study global mechanisms of RNA transcription and processing.
Figures


References
-
- Aach J., Rindone, W., and Church, G.M. 2000. Systematic management and analysis of yeast gene expression data. Genome Res. 10: 431-445. - PubMed
-
- Blattner F.R., Plunkett, G., III, Bloch, C.A., Perna, N.T., Burland, V., Riley, M., Collado-Vides, J., Glasner, J.D., Rode, C.K., Mayhew, G.F., et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 277: 1453-1474. - PubMed
-
- Campbell E.A., Korzheva, N., Mustaev, A., Murakami, K., Nair, S., Goldfarb, A., and Darst, S.A. 2001. Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell 104: 901-912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
