The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan
- PMID: 12566415
- PMCID: PMC2193842
- DOI: 10.1084/jem.20021829
The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan
Abstract
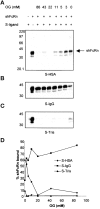
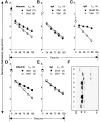
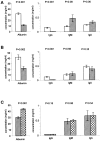
The inverse relationship between serum albumin concentration and its half-life suggested to early workers that albumin would be protected from a catabolic fate by a receptor-mediated mechanism much like that proposed for IgG. We show here that albumin binds FcRn in a pH dependent fashion, that the lifespan of albumin is shortened in FcRn-deficient mice, and that the plasma albumin concentration of FcRn-deficient mice is less than half that of wild-type mice. These results affirm the hypothesis that the major histocompatibility complex-related Fc receptor protects albumin from degradation just as it does IgG, prolonging the half-lives of both.
Figures


References
-
- Peters, T., Jr. 1996. All About Albumin: Biochemistry, Genetics, and Medical Applications. Academic Press, New York.
-
- Freeman, T., and A.H. Gordon. 1965. Albumin catabolism in hypoproteinaemic states studied with 131I-Albumin. Bibl. Haematol. 1108–1115. - PubMed
-
- Cormode, E.J., D.M. Lyster, and S. Israels. 1975. Analbuminemia in a neonate. J. Pediatr. 86:862–867. - PubMed
-
- Waldmann, T.A., and W. Strober. 1969. Metabolism of immunoglobulins. Prog. Allergy. 13:1–110. - PubMed
-
- Schultze, H.E., and J.F. Heremans. 1966. Molecular biology of human proteins: with special reference to plasma proteins. Vol. 1. Nature and Metabolism of Extracellular Proteins. Elsevier, New York. 904 pp.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

