Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion
- PMID: 12566429
- PMCID: PMC2172665
- DOI: 10.1083/jcb.200209095
Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion
Abstract
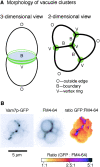
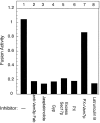
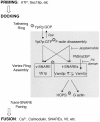
Vacuole tethering, docking, and fusion proteins assemble into a "vertex ring" around the apposed membranes of tethered vacuoles before catalyzing fusion. Inhibitors of the fusion reaction selectively interrupt protein assembly into the vertex ring, establishing a causal assembly hierarchy: (a) The Rab GTPase Ypt7p mediates vacuole tethering and forms the initial vertex ring, independent of t-SNAREs or actin; (b) F-actin disassembly and GTP-bound Ypt7p direct the localization of other fusion factors; (c) The t-SNAREs Vam3p and Vam7p regulate each other's vertex enrichment, but do not affect Ypt7p localization. The v-SNARE Vti1p is enriched at vertices by a distinct pathway that is independent of the t-SNAREs, whereas both t-SNAREs will localize to vertices when trans-pairing of SNAREs is blocked. Thus, trans-SNARE pairing is not required for SNARE vertex enrichment; and (d) The t-SNAREs regulate the vertex enrichment of both G-actin and the Ypt7p effector complex for homotypic fusion and vacuole protein sorting (HOPS). In accord with this hierarchy concept, the HOPS complex, at the end of the vertex assembly hierarchy, is most enriched at those vertices with abundant Ypt7p, which is at the start of the hierarchy. Our findings provide a unique view of the functional relationships between GTPases, SNAREs, and actin in membrane fusion.
Figures





References
-
- Bennett, M.K., N. Calakos, and R.H. Scheller. 1992. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 257:255–259. - PubMed
-
- Bromley, S.K., W.R. Burack, K.G. Johnson, K. Somersalo, T.N. Sims, C. Sumen, M.M. Davis, A.S. Shaw, P.M. Allen, and M.L. Dustin. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375–396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

