The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density
- PMID: 12566432
- PMCID: PMC2172662
- DOI: 10.1083/jcb.200210020
The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density
Abstract
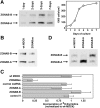
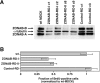
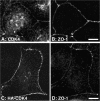
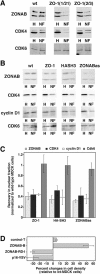
Epithelial tight junctions regulate paracellular permeability, restrict apical/basolateral intramembrane diffusion of lipids, and have been proposed to participate in the control of epithelial cell proliferation and differentiation. Previously, we have identified ZO-1-associated nucleic acid binding proteins (ZONAB), a Y-box transcription factor whose nuclear localization and transcriptional activity is regulated by the tight junction-associated candidate tumor suppressor ZO-1. Now, we found that reduction of ZONAB expression using an antisense approach or by RNA interference strongly reduced proliferation of MDCK cells. Transfection of wild-type or ZONAB-binding fragments of ZO-1 reduced proliferation as well as nuclear ZONAB pools, indicating that promotion of proliferation by ZONAB requires its nuclear accumulation. Overexpression of ZONAB resulted in increased cell density in mature monolayers, and depletion of ZONAB or overexpression of ZO-1 reduced cell density. ZONAB was found to associate with cell division kinase (CDK) 4, and reduction of nuclear ZONAB levels resulted in reduced nuclear CDK4. Thus, our data indicate that tight junctions can regulate epithelial cell proliferation and cell density via a ZONAB/ZO-1-based pathway. Although this regulatory process may also involve regulation of transcription by ZONAB, our data suggest that one mechanism by which ZONAB and ZO-1 influence proliferation is by regulating the nuclear accumulation of CDK4.
Figures




References
-
- Assoian, R.K., and M.A. Schwartz. 2001. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11:48–53. - PubMed
-
- Balda, M.S., J.A. Whitney, C. Flores, S. González, M. Cereijido, and K. Matter. 1996. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 134:1031–1049. - PMC - PubMed
-
- Bargou, R.C., K. Jurchott, C. Wagener, S. Bergmann, S. Metzner, K. Bommert, M.Y. Mapara, K.J. Winzer, M. Dietel, B. Dorken, and H.D. Royer. 1997. Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nat. Med. 3:447–450. - PubMed
-
- Behrens, J. 1999. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev. 18:15–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

