Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge
- PMID: 12569130
- PMCID: PMC195987
- DOI: 10.1101/gad.1044903
Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge
Abstract
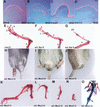
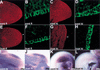
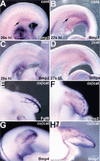
The formation of the apical ectodermal ridge (AER) is critical for the distal outgrowth and patterning of the vertebrate limb. Recent work in the chick has demonstrated that interplay between the Wnt and Fgf signaling pathways is essential in the limb mesenchyme and ectoderm in the establishment and perhaps the maintenance of the AER. In the mouse, whereas a role for Fgfs for AER establishment and function has been clearly demonstrated, the role of Wnt/beta-catenin signaling, although known to be important, is obscure. In this study, we demonstrate that Wnt3, which is expressed ubiquitously throughout the limb ectoderm, is essential for normal limb development and plays a critical role in the establishment of the AER. We also show that the conditional removal of beta-catenin in the ventral ectodermal cells is sufficient to elicit the mutant limb phenotype. In addition, removing beta-catenin after the induction of the ridge results in the disappearance of the AER, demonstrating the requirement for continued beta-catenin signaling for the maintenance of this structure. Finally, we demonstrate that Wnt/beta-catenin signaling lies upstream of the Bmp signaling pathway in establishment of the AER and regulation of the dorsoventral polarity of the limb.
Figures





References
-
- Aberle H, Schwartz H, Kemler R. Cadherin–catenin complex: Protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. - PubMed
-
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., III BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–4461. - PubMed
-
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
-
- Bell SM, Schreiner CM, Scott WJ. The loss of ventral ectoderm identity correlates with the inability to form an AER in the legless hindlimb bud. Mech Dev. 1998;74:41–50. - PubMed
-
- Boussadia O, Kutsch S, Hierholzer A, Delmas V, Kemler R. E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev. 2002;115:53–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
