Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo
- PMID: 12569161
- PMCID: PMC151853
- DOI: 10.1172/JCI16146
Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo
Abstract
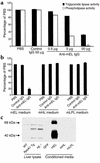
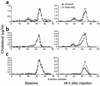
Endothelial lipase (EL) is a recently discovered member of the lipoprotein lipase gene family that hydrolyzes HDL phospholipids ex vivo and reduces HDL cholesterol (HDL-C) levels when overexpressed in vivo in mice. To gain further insight into the physiological role of EL in the metabolism of HDL in vivo, studies were performed in which EL was inhibited in wild-type, hepatic lipase knockout (HL(-/-)), and human apoA-I transgenic mice by intravenous infusion of a polyclonal antibody inhibitory to murine EL. As compared with infusion of a control antibody, infusion of the inhibitory antibody resulted in a 25-60% increase in HDL-C levels in the three mouse models, with the peak HDL-C levels occurring at 48 hours after injection. Inhibition of EL also generated larger HDL particles in the HL(-/-) mice. The clearance of HDL phospholipid was significantly slower in human apoA-I transgenic mice injected with an antibody against murine EL (mEL) than in mice injected with a control antibody. We conclude that inhibition of EL results in increased HDL-C levels and that EL is an important enzyme in the physiological regulation of HDL metabolism.
Figures

Comment in
-
Endothelial lipase: direct evidence for a role in HDL metabolism.J Clin Invest. 2003 Feb;111(3):318-21. doi: 10.1172/JCI17744. J Clin Invest. 2003. PMID: 12569156 Free PMC article. Review. No abstract available.
References
-
- Jaye M, et al. A novel endothelial-derived lipase that modulates HDL metabolism. Nat. Genet. 1999;21:424–428. - PubMed
-
- Hirata K, et al. Cloning of a unique lipase from endothelial cells extends the lipase gene family. J. Biol. Chem. 1999;274:14170–14175. - PubMed
-
- McCoy MG, et al. Characterization of the lipolytic activity of endothelial lipase. J. Lipid Res. 2002;43:921–929. - PubMed
-
- Kuusi T, Kinnunen PK, Nikkila EA. Hepatic endothelial lipase antiserum influences rat plasma low and high density lipoproteins in vivo. FEBS Lett. 1979;104:384–388. - PubMed
-
- Grosser J, Schrecker O, Greten H. Function of hepatic triglyceride lipase in lipoprotein metabolism. J. Lipid Res. 1981;22:437–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

