Metastatic renal carcinoma comprehensive prognostic system
- PMID: 12569375
- PMCID: PMC2747541
- DOI: 10.1038/sj.bjc.6600768
Metastatic renal carcinoma comprehensive prognostic system
Abstract
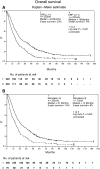
The purpose of the study was to identify a comprehensive prognostic system of pretreatment clinical parameters in 425 patients (pts) with metastatic renal-cell carcinoma treated with different subcutaneous (s.c.) recombinant cytokine-based home therapies in consecutive trials. Treatment consisted of (A) s.c. interferon-alpha 2a (INF-alpha), s.c. interleukin-2 (IL-2) (n=102 pts), (B) s.c. IFN-alpha 2a, s.c. IL-2, and i.v. 5-fluorouracil (5-FU) (n=235 pts) or (C) s.c. IFN-alpha 2a, s.c. IL-2, and i.v. 5-FU combined with p.o. 13-cis-retinoic acid (13cRA) (n=88 pts). Kaplan-Meier survival analysis, log-rank statistics, and Cox regression analysis were employed to identify risk factors and to create a multiple risk factor model. The following pretreatment risk factors were identified by univariate analysis: (1) three and more metastatic sites, (2) presence of liver, lymph node or bone metastases, (3) neutrophil count > or = 6500 cells microl(-1), (4) serum lactate dehydrogenase level (LDH) > or = 220 U l(-1), and (5) serum C-reactive protein level (CRP) > or = 11 mg l(-1). Cox regression analysis with forward stepwise variable selection identified neutrophil count as the major prognostic factor (hazard ratio=1.9, P<0.001), while serum levels of LDH and CRP, time between diagnosis of tumour and onset of metastatic disease, number of metastatic sites, and bone metastases were significant but somewhat less important prognostic variables within the multiple risk factor model (hazard ratio < or = 1.5). Patients were assigned to one of the three risk groups according to cumulative risk defined as the sum of simplified risk s.c.ores for six pretreatment variables. Low-, intermediate-, and high-risk patients achieved a median overall survival of 32+ months (95% CI 24, 43; 5-year survival of 27%), 18+ months (95% CI 15, 20; 5-year survival of 11%), and 8+ months (95% CI 6, 10; 5-year survival of 5%), respectively. These prognostic categories are helpful both in individual patient care and in the assessment of patients entering prospective clinical trials.
Figures


References
-
- Altman DG, Lausen B, Sauerbrei W (1994) Danger of using ‘optimal’ cutpoints in the evaluation of prognostic factors. J Nat Cancer Inst 86(11): 829–835 - PubMed
-
- Atzpodien J, Kirchner H, Illiger HJ, Metzner B, Ukena D, Schott H, Funke PJ, Gramatzki M, von Jürgenson S, Wandert T, Patzelt T, Reitz M. (2001) IL-2 in combination with IFN-α and 5-FU versus tamoxifen in metastatic renal-cell carcinoma: long-term results of a controlled randomised clinical trial. Br J Cancer 85(8): 1130–1136 - PMC - PubMed
-
- Atzpodien J, Kirchner H, Jonas U, Bergmann L, Schott H, Heynemann H, Fornaia P, Loening SA, Roigas J, Müller SC, Westerhausen H, Helbig W, Bodenstein H, Pomer S, Metzner B, Rebmann U, Hofstetter A, Oberneder R, Siebels TI, Wandert T, Patzelt T, Reitz M, DGCIN-Group (2002) 13-CIS-retinoic acid, IFN-alpha2a, IL-2 and chemotherapy in advanced renalcell carcinoma: results of a prospectively randomized trial of The German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN). J Clin Oncol (submitted). - PubMed
-
- Atzpodien J, Korfer A, Franks CR, Knuver-Hopf J, Lopez-Hanninen E, Fischer M, Mohr H, Dallmann I, Hadam M. (1990) Home therapy with recombinant interleukin-2 and interferon-alpha 2b in advanced human malignancies. Lancet; 335: 1509–1512 - PubMed
-
- Blay JY, Rossi JF, Wijdenes J, Menetrier-Caux C, Schemann S, Negrier S, Philip T, Favrot M (1997) Role of interleukin-6 in the paraneoplastic inflammatory syndrome associated with renal-cell carcinoma. Int J Cancer: 72(3): 424–430 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

