Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution
- PMID: 12571021
- PMCID: PMC143632
- DOI: 10.1128/AEM.69.2.987-995.2003
Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution
Erratum in
- Appl Environ Microbiol. 2003 Aug;69(8):5037
Abstract
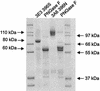
Laccase from Myceliophthora thermophila (MtL) was expressed in functional form in Saccharomyces cerevisiae. Directed evolution improved expression eightfold to the highest yet reported for a laccase in yeast (18 mg/liter). Together with a 22-fold increase in k(cat), the total activity was enhanced 170-fold. Specific activities of MtL mutants toward 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) and syringaldazine indicate that substrate specificity was not changed by the introduced mutations. The most effective mutation (10-fold increase in total activity) introduced a Kex2 protease recognition site at the C-terminal processing site of the protein, adjusting the protein sequence to the different protease specificities of the heterologous host. The C terminus is shown to be important for laccase activity, since removing it by a truncation of the gene reduces activity sixfold. Mutations accumulated during nine generations of evolution for higher activity decreased enzyme stability. Screening for improved stability in one generation produced a mutant more stable than the heterologous wild type and retaining the improved activity. The molecular mass of MtL expressed in S. cerevisiae is 30% higher than that of the same enzyme expressed in M. thermophila (110 kDa versus 85 kDa). Hyperglycosylation, corresponding to a 120-monomer glycan on one N-glycosylation site, is responsible for this increase. This S. cerevisiae expression system makes MtL available for functional tailoring by directed evolution.
Figures


References
-
- Alcalde, M., and T. Bulter. Colorimetric assays for screening laccases. In F. H. Arnold and G. Georgiou (ed.), Directed evolution, vol. 2. Methods in molecular biology series. Humana Press, Totowa, N.J., in press. - PubMed
-
- Arnold, F. H. 2001. Combinatorial and computational challenges for biocatalyst design. Nature 409:253-257. - PubMed
-
- Arnold, F. H., and A. A. Volkov. 1999. Directed evolution of biocatalysts. Curr. Opin. Chem. Biol. 3:54-59. - PubMed
-
- Bajpai, P. 1999. Application of enzymes in the pulp and paper industry. Biotechnol. Prog. 15:147-157. - PubMed
-
- Bauer, R., and C. O. Rupe. 1971. Use of syringaldazine in a photometric method for estimating “free” chlorine in water. Anal. Chem. 43:421-425.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

