Three transporters mediate uptake of glycine betaine and carnitine by Listeria monocytogenes in response to hyperosmotic stress
- PMID: 12571024
- PMCID: PMC143676
- DOI: 10.1128/AEM.69.2.1013-1022.2003
Three transporters mediate uptake of glycine betaine and carnitine by Listeria monocytogenes in response to hyperosmotic stress
Abstract
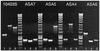
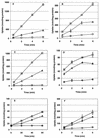
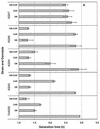
The uptake and accumulation of the potent osmolytes glycine betaine and carnitine enable the food-borne pathogen Listeria monocytogenes to proliferate in environments of elevated osmotic stress, often rendering salt-based food preservation inadequate. To date, three osmolyte transport systems are known to operate in L. monocytogenes: glycine betaine porter I (BetL), glycine betaine porter II (Gbu), and a carnitine transporter OpuC. We investigated the specificity of each transporter towards each osmolyte by creating mutant derivatives of L. monocytogenes 10403S that possess each of the transporters in isolation. Kinetic and steady-state osmolyte accumulation data together with growth rate experiments demonstrated that osmotically activated glycine betaine transport is readily and effectively mediated by Gbu and BetL and to a lesser extent by OpuC. Osmotically stimulated carnitine transport was demonstrated for OpuC and Gbu regardless of the nature of stressing salt. BetL can mediate weak carnitine uptake in response to NaCl stress but not KCl stress. No other transporter in L. monocytogenes 10403S appears to be involved in osmotically stimulated transport of either osmolyte, since a triple mutant strain yielded neither transport nor accumulation of glycine betaine or carnitine and could not be rescued by either osmolyte when grown under elevated osmotic stress.
Figures





References
-
- Angelidis, A. S., L. T. Smith, and G. M. Smith. 2002. Elevated carnitine accumulation by Listeria monocytogenes impaired in glycine betaine transport is insufficient to restore wild-type cryotolerance in milk whey. Int. J. Food Microbiol. 75:1-9. - PubMed
-
- Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. - PubMed
-
- Buchanan, R. L., and L. A. Klawitter. 1990. Effects of temperature and oxygen on the growth of Listeria monocytogenes at pH 4.5. J. Food Sci. 55:1754-1756.
-
- Cole, M. B., M. V. Jones, and C. Holyoak. 1990. The effect of pH, salt concentration and temperature on the survival and growth of Listeria monocytogenes. J. Appl. Bacteriol. 69:63-72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

