Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes
- PMID: 12571356
- PMCID: PMC149943
- DOI: 10.1073/pnas.0435906100
Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes
Abstract
Liposomes modified with TAT peptide (TATp-liposomes) showed fast and efficient translocation into the cell cytoplasm with subsequent migration into the perinuclear zone. TATp-liposomes containing a small quantity (<or=10 mol %) of a cationic lipid formed firm noncovalent complexes with DNA. Here, we present results demonstrating both in vitro and in vivo transfection with TATp-liposome-DNA complexes. Mouse NIH/3T3 fibroblasts and rat H9C2 cardiomyocytes were transfected with such complexes in vitro. The transfection with the TATp-liposome-associated pEGFP-N1 plasmid encoding for the green fluorescent protein (GFP) was high, whereas the cytotoxicity was lower than that of commonly used cationic lipid-based gene-delivery systems. Intratumoral injection of TATp-liposome-DNA complexes into the Lewis lung carcinoma tumor of mice also resulted in an expression of GFP in tumor cells. This transfection system should be useful for various protocols of cell treatment in vitro or ex vivo as well as for localized in vivo gene therapy.
Figures
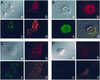


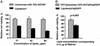

References
-
- McCormic F. Nat Rev Cancer. 2001;1:130–141. - PubMed
-
- Searle P F, Spiers I, Simpson J, James N D. Drug Delivery Syst Sci. 2002;1:5–13.
-
- Chang L J, He J. Curr Opin Mol Ther. 2001;3:468–475. - PubMed
-
- Buchschacher G L, Jr, Wong-Staal F. Hum Gene Ther. 2001;12:1013–1019. - PubMed
-
- Dornburg R, Pomerantz R J. Adv Pharmacol. 2000;49:229–261. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

