The deoxyxylulose phosphate pathway of isoprenoid biosynthesis: studies on the mechanisms of the reactions catalyzed by IspG and IspH protein
- PMID: 12571359
- PMCID: PMC149876
- DOI: 10.1073/pnas.0337742100
The deoxyxylulose phosphate pathway of isoprenoid biosynthesis: studies on the mechanisms of the reactions catalyzed by IspG and IspH protein
Abstract
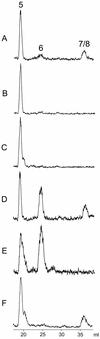
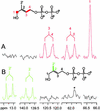
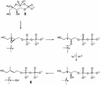
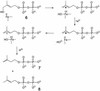
Earlier in vivo studies have shown that the sequential action of the IspG and IspH proteins is essential for the reductive transformation of 2C-methyl-d-erythritol 2,4-cyclodiphosphate into dimethylallyl diphosphate and isopentenyl diphosphate via 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate. A recombinant fusion protein comprising maltose binding protein and IspG protein domains was purified from a recombinant Escherichia coli strain. The purified protein failed to transform 2C-methyl-d-erythritol 2,4-cyclodiphosphate into 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate, but catalytic activity could be restored by the addition of crude cell extract from an ispG-deficient E. coli mutant. This indicates that auxiliary proteins are required, probably as shuttles for redox equivalents. On activation by photoreduced 10-methyl-5-deaza-isoalloxazine, the recombinant protein catalyzed the formation of 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate from 2C-methyl-d-erythritol 2,4-cyclodiphosphate at a rate of 1 nmol x min(-1) x mg(-1). Similarly, activation by photoreduced 10-methyl-5-deaza-isoalloxazine enabled purified IspH protein to catalyze the conversion of 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate into a 6:1 mixture of isopentenyl diphosphate and dimethylallyl diphosphate at a rate of 0.4 micromol x min(-1) x mg(-1). IspH protein could also be activated by a mixture of flavodoxin, flavodoxin reductase, and NADPH at a rate of 3 nmol x min(-1) x mg(-1). The striking similarities of IspG and IspH protein are discussed, and plausible mechanistic schemes are proposed for the two reactions.
Figures


References
-
- Bach T J. Lipids. 1995;30:191–202. - PubMed
-
- Bloch K. Steroids. 1992;57:378–382. - PubMed
-
- Bochar D A, Friesen J A, Stauffacher C V, Rodwell V W. In: Comprehensive Natural Product Chemistry. Cane D, editor. Vol. 2. Oxford: Pergamon; 1999. pp. 15–44.
-
- Qureshi N, Porter J W. In: Biosynthesis of Isoprenoid Compounds. Porter J W, Spurgeon S L, editors. Vol. 1. New York: Wiley; 1981. pp. 47–94.
-
- Slater E, MacDonalds J S. Drugs. 1988;36:72–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

