Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6
- PMID: 12574114
- PMCID: PMC145440
- DOI: 10.1093/emboj/cdg080
Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6
Abstract
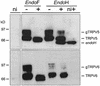
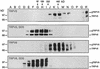
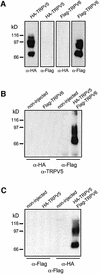
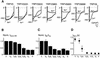
The molecular assembly of the epithelial Ca(2+) channels (TRPV5 and TRPV6) was investigated to determine the subunit stoichiometry and composition. Immunoblot analysis of Xenopus laevis oocytes expressing TRPV5 and TRPV6 revealed two specific bands of 75 and 85-100 kDa, corresponding to the core and glycosylated proteins, respectively, for each channel. Subsequently, membranes of these oocytes were sedimented on sucrose gradients. Immuno blotting revealed that TRPV5 and TRPV6 complexes migrate with a mol. wt of 400 kDa, in line with a tetrameric structure. The tetrameric stoichiometry was confirmed in an electrophysiological analysis of HEK293 cells co-expressing concatemeric channels together with a TRPV5 pore mutant that reduced Cd(2+) sensitivity and voltage-dependent gating. Immuno precipitations using membrane fractions from oocytes co-expressing TRPV5 and TRPV6 demonstrated that both channels can form heteromeric complexes. Expression of all possible heterotetrameric TRPV5/6 complexes in HEK293 cells resulted in Ca(2+) channels that varied with respect to Ca(2+)-dependent inactivation, Ba(2+) selectivity and pharmacological block. Thus, Ca(2+)-transporting epithelia co-expressing TRPV5 and TRPV6 can generate a pleiotropic set of functional heterotetrameric channels with different Ca(2+) transport kinetics.
Figures




Similar articles
-
Interaction of the epithelial Ca2+ channels TRPV5 and TRPV6 with the intestine- and kidney-enriched PDZ protein NHERF4.Pflugers Arch. 2006 Jul;452(4):407-17. doi: 10.1007/s00424-006-0051-z. Epub 2006 Mar 25. Pflugers Arch. 2006. PMID: 16565876
-
Functional expression of the epithelial Ca(2+) channels (TRPV5 and TRPV6) requires association of the S100A10-annexin 2 complex.EMBO J. 2003 Apr 1;22(7):1478-87. doi: 10.1093/emboj/cdg162. EMBO J. 2003. PMID: 12660155 Free PMC article.
-
Molecular determinants in TRPV5 channel assembly.J Biol Chem. 2004 Dec 24;279(52):54304-11. doi: 10.1074/jbc.M406222200. Epub 2004 Oct 15. J Biol Chem. 2004. PMID: 15489237
-
The epithelial calcium channels, TRPV5 & TRPV6: from identification towards regulation.Cell Calcium. 2003 May-Jun;33(5-6):497-507. doi: 10.1016/s0143-4160(03)00065-4. Cell Calcium. 2003. PMID: 12765695 Review.
-
The epithelial calcium channels TRPV5 and TRPV6: regulation and implications for disease.Naunyn Schmiedebergs Arch Pharmacol. 2005 Apr;371(4):295-306. doi: 10.1007/s00210-005-1021-2. Naunyn Schmiedebergs Arch Pharmacol. 2005. PMID: 15747113 Review.
Cited by
-
Expression of Transient Receptor Potential Vanilloid (TRPV) channels in different passages of articular chondrocytes.Int J Mol Sci. 2012;13(4):4433-4445. doi: 10.3390/ijms13044433. Epub 2012 Apr 10. Int J Mol Sci. 2012. PMID: 22605988 Free PMC article.
-
The role of the TRPV6 channel in cancer.J Physiol. 2012 Mar 15;590(6):1369-76. doi: 10.1113/jphysiol.2011.225862. Epub 2012 Feb 13. J Physiol. 2012. PMID: 22331416 Free PMC article. Review.
-
A specific subset of transient receptor potential vanilloid-type channel subunits in Caenorhabditis elegans endocrine cells function as mixed heteromers to promote neurotransmitter release.Genetics. 2007 Jan;175(1):93-105. doi: 10.1534/genetics.106.065516. Epub 2006 Oct 22. Genetics. 2007. PMID: 17057248 Free PMC article.
-
Structure-function analysis of TRPV channels.Naunyn Schmiedebergs Arch Pharmacol. 2005 Apr;371(4):285-94. doi: 10.1007/s00210-005-1053-7. Naunyn Schmiedebergs Arch Pharmacol. 2005. PMID: 15889240 Review.
-
Forward genetic analysis reveals multiple gating mechanisms of TRPV4.J Biol Chem. 2010 Jun 25;285(26):19884-90. doi: 10.1074/jbc.M110.113936. Epub 2010 Apr 27. J Biol Chem. 2010. PMID: 20424166 Free PMC article.
References
-
- Bahner M., Sander,P., Paulsen,R. and Huber,A. (2000) The visual G protein of fly photoreceptors interacts with the PDZ domain assembled INAD signaling complex via direct binding of activated Gαq to phospholipase cβ. J. Biol. Chem., 275, 2901–2904. - PubMed
-
- Bodding M., Wissenbach,U. and Flockerzi,V. (2002) The recombinant human TRPV6 channel functions as Ca2+ sensor in human embryonic kidney and rat basophilic leukemia cells. J. Biol. Chem., 277, 36656–36664. - PubMed
-
- Brown A.J., Finch,J. and Slatopolsky,E. (2002) Differential effects of 19-nor-1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 on intestinal calcium and phosphate transport. J. Lab. Clin. Med., 139, 279–284. - PubMed
-
- Clapham D.E., Runnels,L.W. and Strubing,C. (2001) The TRP ion channel family. Nat. Rev. Neurosci., 2, 387–396. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

