Galpha(s) is palmitoylated at the N-terminal glycine
- PMID: 12574119
- PMCID: PMC145455
- DOI: 10.1093/emboj/cdg095
Galpha(s) is palmitoylated at the N-terminal glycine
Abstract
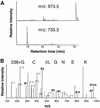
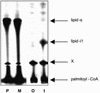
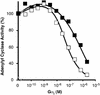
Covalent lipid attachments are essential co- and post-translational modifications for signalling proteins. Galpha(s), the alpha-subunit of the heterotrimeric G protein that activates adenylyl cyclase, is known to be palmitoylated at the third N-terminal amino acid, a cysteine. Palmitoylation is involved in anchoring Galpha(s) to the membrane by increasing its intrinsic hydrophobicity. We identified by mass spectrometry a second, functionally even more important, covalent modification. It consists of another palmitoyl residue attached to the preceding glycine (Gly(2)). Palmitoylation at this position has profound consequences for levels of signal transduction. It sensitizes the cell up to 200-fold for adenylyl cyclase-stimulating agents. The inhibitory inputs mediated by Galpha(i) are downregulated to <10%. Thereby, Gly(2)-palmitoylation of Galpha(s) relieves cellular stimulation at the level of adenylyl cyclase whereas it renders the inhibitory modulation via Galpha(i) more difficult.
Figures



References
-
- Berthiaume L. and Resh,M.D. (1995) Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J. Biol. Chem., 270, 22399–22405. - PubMed
-
- Beyermann M., Fechner,K., Furkert,J., Krause,E. and Bienert,M. (1996) A single-point slight alteration set as a tool for structure-activity relationship studies of ovine corticotropin releasing factor. J. Med. Chem., 39, 3324–3330. - PubMed
-
- Casey P.J. (1994) Lipid modifications of G proteins. Curr. Opin. Cell Biol., 6, 219–225. - PubMed
-
- Casey P.J. (1995) Protein lipidation in cell signaling. Science, 268, 221–225. - PubMed
-
- Chamoun Z., Mann,R.K., Nellen,D., von Kessler,D.P., Bellotto,M., Beachy,P.A. and Basler,K. (2001) Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science, 293, 2080–2084. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

